In the previous section, we saw the nomenclature and isomerism in alkanes. In this section, we will see some advanced examples.
Example 1:
Write the IUPAC name of the structure shown in fig.13.13 below:
 |
| Fig.13.13 |
Solution:
• We have seen the 8 rules in section 12.3
1. Applying rule 1, we see that, there are six C atoms in the main chain.
2. Applying rule 2, we see that, 'hex' must be used.
3. Applying rule 3, we get hexane.
4. Applying rule 4, we see that, there are two branches: methyl and ethyl
5. Applying rule 5, we see that, the correct way of numbering is indeed from left to right as shown in fig.13.13(a).
• If we number the C atoms from left to right as in fig.b, the branches will get the numbers 2,4. The sum is (2+4) = 6.
• If we number the C atoms from
right to left, the branches will get the numbers 3,5. The sum is (3+5) = 8, which is
larger and hence not acceptable.
6. Applying rule 6, we get: 2-methyl and 4-ethyl
7. Applying rule 7, we get: 4-ethyl-2-methyl
• This is because, 'e' comes before 'm' in the alphabetical listing.
8. Applying rule 8, we get: 4-Ethyl-2-methylhexane.
Example 2:
Write the IUPAC name of the structure shown in fig.13.14 below:
 |
| Fig.13.14 |
Solution:
In this problem, there are branches within a branch. So we must be ready to apply the rules that we saw in section 12.4 also. Fortunately, there is a common name available for that branch. So we can first name the main carbon chain.
1. Applying rule 1, we see that, there are eight C atoms in the main chain.
2. Applying rule 2, we see that, 'oct' must be used.
3. Applying rule 3, we get octane.
4. Applying rule 4, we see that, there are four branches: two ethyl branches, one methyl branch and one isopropyl branch.
5. Applying rule 5, we see that, the correct way of numbering is
from right to left as shown in fig.13.14(b).
• If we number the C atoms from
right to left, the branches will get the numbers 3,3,4,5. The sum is (3+3+4+5) = 15.
• If we number the C atoms from
left to right (fig.a), the branches will get the numbers 4,5,6,6. The sum is (4+5+6+6) = 21, which is
larger and hence not acceptable.
6. Applying rule 6, we get: 3-ethyl, 3-ethyl, 4-methyl and 5-isopropyl
7. Applying rule 7, 7A and 7B, we get: 3,3-diethyl-5-isopropyl-4-methyl
• This can be explained in 2 steps:
(i) 'e' comes before 'i' and 'm' in the alphabetical listing.
'di' is not considered as part of the name. So 'd' should not be considered for alphabetical listing.
(ii) 'i' comes before 'm' in the alphabetical listing.
'iso' is considered as part of the name.
8. Applying rule 8, we get: 3,3-diethyl-5-isopropyl-4-methyloctane.
Example 3:
Write the IUPAC name of the structure shown in fig.13.15 below:
 |
| Fig.13.15 |
Solution:
In this problem, there are branches within a branches. So we must be ready to apply the rules that we saw in section 12.4 also. Fortunately, there are common names available for those branches. So we can first name the main carbon chain.
1. Applying rule 1, we see that, there are ten C atoms in the main chain.
2. Applying rule 2, we see that, 'dec' must be used.
3. Applying rule 3, we get decane.
4. Applying rule 4, we see that, there are two branches: one isopropyl branch and and one sec-butyl branch.
5. Applying rule 5, we see that, the correct way of numbering is from left to right as shown in fig.13.15(a).
• If we number the C atoms from left to right, the branches will get the numbers 4,5. The sum is (4+5) = 9.
• If we number the C atoms from right to left (fig.b), the branches will get the numbers 6,7. The sum is (6+7) = 13, which is larger and hence not acceptable.
6. Applying rule 6, we get: 4-isopropyl and 5-sec-butyl
7. Applying rule 7, 7A and 7B, we get: 5-sec-butyl-4-isopropyl
• This can be explained in 3 steps:
(i) 'b' comes before 'i' in the alphabetical listing.
(ii) 'sec' is not considered as part of the name. So 's' should not be considered for alphabetical listing.
(iii) 'iso' is considered as part of the name.
8. Applying rule 8, we get: 5-sec-butyl-4-isopropyl decane.
Example 4:
Write the IUPAC name of the structure shown in fig.13.16 below:
 |
| Fig.13.16 |
Solution:
In this problem, there are branches within a branch. So we must be ready to apply the rules that we saw in section 12.4 also. The branch do not have any common name. So we first name the branch.
1. Applying rule 1, we see that, there are three C atoms.
2. Applying rule 2, we see that, 'prop' must be used.
3. Applying rule 3, we get propane.
4. Applying rule 4, we see that, there are two branches: two methyl groups.
5. Applying rule 5, we see that, the correct way of numbering is
from the main branch as shown in fig.13.16(a).
6. Applying rule 6, we get: 2-methyl and 2-methyl
7. Applying rule 7, we get: 2,2-dimethyl
8. Applying rule 8, we get: 2,2-dimethylpropyl.
• This name must be written within parenthesis.
Now we can name the main chain.
1. Applying rule 1, we see that, there are nine C atoms.
2. Applying rule 2, we see that, 'non' must be used.
3. Applying rule 3, we get nonane.
4. Applying rule 4, we see that, there is only one branch.
5. Applying rule 5, we see that, numbering can be done in both ways. Both will give the number 5.
6. Applying rule 6 we get: 5-(2,2-dimethylpropyl)
7. Applying rule 7 is not required because there is only one branch.
8. Applying rule 8, we get: 5-(2,2-dimethylpropyl)nonane.
Example 5:
Write the IUPAC name of the structure shown in fig.13.17 below:
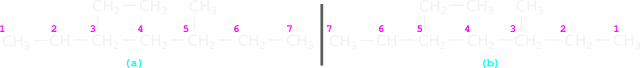 |
| Fig.13.17 |
• We have seen the 8 rules in section 12.3
1. Applying rule 1, we see that, there are seven C atoms in the main chain.
2. Applying rule 2, we see that, 'hept' must be used.
3. Applying rule 3, we get heptane.
4. Applying rule 4, we see that, there are two branches: methyl and ethyl
5. Applying rule 5:
♦ We see that, the numbering can be done in both ways.
♦ In both fig. a and b, the numbers are 3 and 5.
• So we apply rule 5A:
If two branches are present in equivalent positions, then the lower number should be given to that branch which comes first in the alphabetical order.
♦ Here, ethyl comes first in the alphabetical order.
♦ Numbering in fig.a is correct.
6. Applying rule 6, we get: 3-ethyl and 5-methyl
7. Applying rule 7, we get: 3-ethyl-5-methyl
• This is because, 'e' comes before 'm' in the alphabetical listing.
8. Applying rule 8, we get: 3-Ethyl-5-methylheptane.
Solved example 13.3
Write the IUPAC names of the following compounds:
(i) (CH3)3CCH2C(CH3)3
(ii) (CH3)2C(C2H5)2
(iii) tetra-tert-butylmethane
Solution:
Part (i):
• We are given the condensed formula. Based on the condensed formula, we can draw the structural formula. It is shown in fig.13.18 below:
 |
| Fig.13.18 |
• We have seen the 8 rules in section 12.3
1. Applying rule 1, we see that, there are five C atoms in the main chain.
2. Applying rule 2, we see that, 'pent' must be used.
3. Applying rule 3, we get pentane.
4. Applying rule 4, we see that, there are four branches: four methyl branches.
5. Applying rule 5:
♦ We see that, the numbering can be done in both ways.
♦ In both fig. a and b, the numbers are 2 and 4.
6. Applying rule 6, we get: 2-methy, 2-methyl, 4-methy, 4-methyl
7. Applying rule 7, we get: 2,2,4,4-tetramethyl
8. Applying rule 8, we get: 2,2,4,4-Tetramethylpentane.
Part (ii):
• We are given the condensed formula. Based on the
condensed formula, we can draw the structural formula. It is shown in
fig.13.19 below:
 |
| Fig.13.19 |
• We have seen the 8 rules in section 12.3
1. Applying rule 1, we see that, there are five C atoms in the main chain.
2. Applying rule 2, we see that, 'pent' must be used.
3. Applying rule 3, we get pentane.
4. Applying rule 4, we see that, there are two branches: two methyl branches.
5. Applying rule 5:
♦ We see that, the numbering can be done in both ways.
♦ In both fig. a and b, the number is 3.
6. Applying rule 6, we get: 3-methy, 3-methyl
7. Applying rule 7, we get: 2,2-dimethyl
8. Applying rule 8, we get: 2,2-Dimethylpentane.
Part (iii):
• We have seen the 8 rules in section 12.3
• In this problem, there are branches within a branch. So we must be ready to apply the rules that we saw in section 12.4 also.
• We are given the common name tetra-tert-butylmethane.
♦ This is similar to the common name: tetra-chloromethane.
• In tetrachloromethane, the four H atoms of methane are replaced by four Cl atoms.
• In the same way, in tetra-tert-butylmethane, the four H atoms of methane are replaced by four tert-butyl groups.
• We saw the structure of tert-butyl in an earlier section [see fig.12.33 in section section 12.4]. It is shown again in fig.13.20(a) below:
 |
| Fig.13.20 |
• Based on the structure of tert-butyl, the structure of tetra-tert-butylmethane will be as shown in fig.b. Now we can write the IUPAC name.
1. Applying rule 1, we see that, there are five C atoms in the main chain.
2. Applying rule 2, we see that, 'pent' must be used.
3. Applying rule 3, we get pentane.
4. Applying rule 4, we see that, there are six branches: four methyl branches and two tert-butyl branches.
5. Applying rule 5:
♦ We see that, the numbering can be done in two ways.
♦ In both fig. b and c, the numbers are 2, 3 and 4.
6. Applying rule 6, we get: 2-methy, 2-methyl, 4-methy, 4-methyl, 3-tert-butyl, 3-tert-butyl
7. Applying rule 7, we get: 3,3-di-tert-butyl-2,2,4,4-tetramethyl
• Remember that, 'tetra' is not considered as part of name. So 't' cannot be considered in the alphabetical listing.
8. Applying rule 8, we get: 3,3-Di-tert-butyl-2,2,4,4-tetramethylpentane.
• In the above discussion, we were given the structures of hydrocarbons. We wrote the corresponding IUPAC names.
• We must be able to do the reverse also. That is., we will be given the IUPAC names. We must draw the corresponding structures.
• Let us see an example:
Draw the structure of 3-Ethyl-2,2-dimethylpentane
Solution:
1. In the given name, we have ‘pent’ as the root. Then there will be five C atoms in the longest chain. So we first draw a chain of five C atoms. This is shown in fig.13.21(a) below:
 |
| Fig.13.21 |
2. Next we number the C atoms from 1 to 5. This is shown in fig.b
3. Attaching the branches:
‘3-Ethyl’ indicates that, there is an ethyl group at the C atom number 3
So we draw an ethyl group at that C atom.
‘2,2-dimethyl’ indicates that, there are two methyl groups at the C atom number 2
So we draw two methyl groups at that C atom
This is shown in fig.c
4. Now we put the required number of H atoms to satisfy the valencies of the C atoms.
Thus we get the final structure shown in fig.d
Solved example 13.4
Write the structural formulas for the following compounds:
(i) 3,4,4,5-Tetramethylheptane
(ii) 2,5-dimethylhexane
Solution:
Part (i):
1. In the given name, we have ‘hept’ as the root. Then there will be
seven C atoms in the longest chain. So we first draw a chain of seven C
atoms. This is shown in fig.13.22(a) below:
 |
| Fig.13.22 |
2. Next we number the C atoms from 1 to 7. This is shown in fig.b
3. Attaching the branches:
‘3,4,4,5-tetramethyl’ indicates that, there are four methyl groups at the three C atoms as written below:
♦ One methyl group at C atom number 3
♦ Two methyl groups at C atom number 4
♦ One methyl group at C atom number 5
• This is shown in fig.c
4. Now we put the required number of H atoms to satisfy the valencies of the C atoms.
• Thus we get the final structure shown in fig.d
Part (ii):
1. In the given name, we have ‘hex’ as the root. Then there will be
six C atoms in the longest chain. So we first draw a chain of six C
atoms. This is shown in fig.13.23(a) below.
 |
| Fig.13.23 |
2. Next we number the C atoms from 1 to 6. This is shown in fig.b
3. Attaching the branches:
‘2,5-dimethyl’ indicates that, there are two methyl groups at the two C atoms as written below:
♦ One methyl group at C atom number 2
♦ One methyl group at C atom number 5
• This is shown in fig.c
4. Now we put the required number of H atoms to satisfy the valencies of the C atoms.
• Thus we get the final structure shown in fig.d
Solved example 13.5
Write structures for each of the following compounds. Why are the given names incorrect. Write the correct IUPAC names.
(i) 2-Ethylpentane
(ii) 5-Ethyl-3-methylheptane
Solution:
Part (i):
1. In the given name, we have ‘pent’ as the root. Then there will be
five C atoms in the longest chain. So we first draw a chain of five C
atoms. This is shown in fig.13.24(a) below:
 |
| Fig.13.24 |
2. Next we number the C atoms from 1 to 5. This is shown in fig.b
3. Attaching the branches:
‘2-ethyl indicates that, there is an ethyl group at the C atom number 2.
• This is shown in fig.c
4. Now we put the required number of H atoms to satisfy the valencies of the C atoms.
• Thus we get the final structure shown in fig.d
5. For this structure, the name 3-Ethylpentane is wrong. The correct name can be obtained in 4 steps:
(i) For this structure, the C atoms should be numbered as shown in fig.e
(ii) Now we see that, there are six C atoms in the longest chain.
(iii) We also see that, the branch is methyl, not ethyl. The branch is at the third C atom.
(iv) So the correct IUPAC name is: 3-Methylhexane.
Part (ii):
1. In the given name, we have ‘hept’ as the root. Then there will be
seven C atoms in the longest chain. So we first draw a chain of seven C
atoms. This is shown in fig.13.25(a) below:
 |
| Fig.13.25 |
2. Next we number the C atoms from 1 to 7. This is shown in fig.b
3. Attaching the branches:
♦ ‘5-ethyl' indicates that, there is an ethyl group at the C atom number 5.
♦ ‘3-methyl' indicates that, there is a methyl group at the C atom number 3.
• This is shown in fig.c
4. Now we put the required number of H atoms to satisfy the valencies of the C atoms.
• Thus we get the final structure shown in fig.d
5. For this structure, the name 5-Ethyl-3-methylheptane is wrong. The correct name can be obtained in 4 steps:
(i) For this structure, the C atoms can be numbered as shown in fig.e also
(ii) Both methods of numbering will give the same numbers 3 and 5.
• That means, the branches are at equivalent positions.
(iii) So we must apply rule 5A. [see section 12.3]
Then 'ethyl' gets the lower number because, it comes first in the alphabetical order.
(iv) So the numbering in fig.e is the correct method.
• Based on this numbering, the correct IUPAC name is: 3-Ethyl-5-methylheptane.
In the next section we will see preparation of alkanes.
Copyright©2022 Higher secondary chemistry.blogspot.com
No comments:
Post a Comment