In the previous section, we saw bond-line formula. In this section we will see classification of organic compounds. Later in this section, we will see nomenclature also.
Classification of organic compounds
• Organic compounds can be broadly classified into two categories:
(i) Open chain compounds (ii) Closed chain compounds
The basis of this classification can be written in 3 steps:
1. We know that, carbon atoms can bond together to form long chains.
2. If there is no bond between the first and last C atoms in a chain, then that chain is an open chain compound.
3. If the first and last C atoms in that chain are bonded together, then that chain is a closed chain compound.
| • Open chain compounds are also known as Acyclic compounds. ♦ They have yet another name: Aliphatic compounds. • Closed chain compounds are also known as cyclic compounds. ♦ They have yet another name: Ring compounds. |
• This broad classification is shown in fig.12.20 below:
 |
| Fig.12.20 |
• Closed chain compounds are again classified into two categories:
(i) Homocyclic compounds (ii) Heterocyclic compounds
• The basis of this classification can be written in 3 steps:
1. Consider the junctions in a closed chain.
2. If all junctions are formed by C atoms, it is called a homocyclic compound. See fig.12.21(i) below.
3. If one or more junctions is formed by atoms other than C, it is called a heterocyclic compound. See fig.12.21 (ii) below.
 |
| Fig.12.21 |
 |
| Fig.12.22 |
Functional groups
• The presence of certain atoms or groups of atoms, impart certain characteristic properties to compounds. Such atoms or groups are called functional groups.We have seen some basics about this topic in our earlier chemistry classes (Details here).
• We do not have to worry about how the group is formed, or what happens inside the group. All we need to know is this:
One H and the bond (‘-’) associated with that H, is removed from the original compound. A functional group and the bond (‘-’) associated with that group takes the position of the removed H.
• Some examples of functional groups are:
♦ Hydroxyl group (-OH)
♦ Aldehyde group (-CHO)
♦ Carboxylic acid group (-COOH)
• Recall how the valencies are satisfied when one H is removed and a hydroxyl group takes it’s place. (See fig.14.62 from our earlier chemistry lessons)
• Recall how the valencies are satisfied when one H is removed and an aldehyde group takes it’s place. (See fig.14.65 from our earlier chemistry lessons)
Homologous series
• We have seen the basic details about homologous series in our earlier chemistry classes.
• We have seen the details about following four points:
(i) Each series can be represented by a general molecular formula.
♦ Alkanes have the general formula CnH2n+2
♦ Alkenes have the general formula CnH2n
♦ Alkynes have the general formula CnH2n-2
(ii) Successive members differ from each other by a -CH2 unit
(iii) Alkanes, Alkenes, Alkynes, Haloalkanes, alkanols etc., are examples of homologous series.
(iv) Members of the homologous series are called homologues.
Nomenclature of organic compounds
• In olden days, the number of organic compounds were not very large. So names were given based on the origin of the compound or properties of the compound.
• Let us see some examples:
♦ Citric acid got it’s name because it is found in citrus fruits.
♦ Formic acid got it’s name because it is found in red ants.
✰ (The Latin word for ant is formica)
♦ Buckminsterfullerene (a cluster of carbon atoms) got it’s name because, it’s shape resembles the domes designed by the famous architect R. Buckminster Fuller.
• Such names are called common names. They are also called trivial names.
• With the advance of science, millions of organic compounds came to be known to man. Naturally, there will be millions of names. So it is not possible to name them based on properties or origins.
• We need a systematic naming procedure. The advantage of a systematic procedure can be written in two steps:
(i) The name of any compound can be written based on the name of it’s preceding compound in the series.
(ii) A person who reads or listens to the name of a compound, will be able to understand the structure of the compound and also the series to which the compound belongs.
• Such a system is developed by the IUPAC (International Union of Pure and Applied Chemistry)
• This system is known as the IUPAC system of nomenclature. It is the system followed by scientists and engineers all over the world.
• We have seen the basic details about this system in our earlier chemistry classes. In this section we will see some advanced details.
• Let us write the basic rules of the system.
• While writing them, we will specially mention those rules which are already known to us.
• We will also specially mention those rules which are new to us.
Rule 1. Compounds containing carbon and hydrogen only, are called hydrocarbons. Firstly, we need to identify the parent hydrocarbon chain. For example, the yellow rectangle in fig.12.23(a) below, shows the parent chain in that molecule.
 |
| Fig.12.23 |
♦ In fig.a, the selected chain has nine C atoms.
♦ In fig.b, the selected chain has eight C atoms.
• So fig.a shows the correct parent chain.
• This rule is already known to us.
Rule 2. After counting the number of C atoms in the parent chain, use the following prefixes:
♦ ‘meth’ for one C atom
♦ ‘eth’ for two C atoms
♦ ‘prop’ for three C atoms
♦ ‘but’ for four C atoms . . . so on . . .
• This rule is already known to us.
• For our present case, we must use 'non'.
Rule 3. If all the carbon-carbon bonds in the parent chain are single bonds, then it is a saturated hydrocarbon.
♦ The parent chain is then an alkane. Use the suffix ‘ane’.
• If one or more carbon-carbon bonds in the parent chain are double bonds, then it is an unsaturated hydrocarbon.
♦ The parent chain is then an alkene. Use the suffix ‘ene’.
• If one or more carbon-carbon bonds in the parent chain are triple bonds, then it is an unsaturated hydrocarbon.
♦ The parent chain is then an alkyne. Use the suffix ‘yne’.
• This rule is already known to us.
• The parent hydrocarbon in our present case is an alkane. So we use 'ane' and thus get: nonane
Rule 4. Identify the branches. The green rectangles in fig.12.24 below indicate the branches.
 |
| Fig.12.24 |
• The branches are called alkyl groups.
• An alkyl group is obtained by removing a H atom from an alkane.
♦ So it is easy to name any alkyl group.
♦ Remove ‘ane’ and put ‘yl’ in it’s place.
• Thus we have:
♦ CH4 (methane) becomes -CH3 (methyl group)
♦ CH3CH3 (ethane) becomes -CH2CH3 (ethyl group)
♦ CH3CH2CH3 (propane) becomes -CH2CH2CH3 (propyl group)
• This rule is already known to us.
• In our present case,
♦ The branch in the small green rectangle is methyl group.
♦ The branch in the large green rectangle is ethyl group.
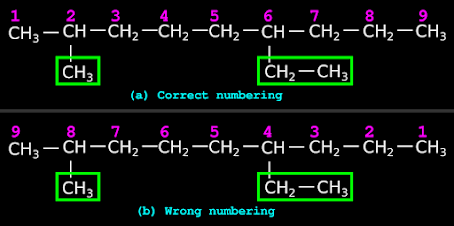
Rule 5. We have already counted the number of C atoms in the parent chain. It was done so as to give the correct name to the parent chain.
• Now, we have to write those counting numbers by the side of the C atoms in the main chain.
• The counting numbers must be written in such a way that, the C atoms carrying the branches get the lowest possible numbers.
• This rule is already known to us.
• In our present case, the correct way of numbering is shown in fig.12.25(a) below.
♦ In fig.a, the branches get the numbers 2 and 6
♦ In fig.b, the branches get larger numbers 4 and 8
♦ So fig.a shows the correct method of numbering.
 |
| Fig.12.25 |
Rule 6. Next we write the names of the branches.
• The names should be written in the correct format:
Position of the branch[no space]hyphen[no space]name of branch
• This rule is already known to us.
• In our present case, the names of the branches are:
♦ 2-methyl
♦ 6-ethyl
Rule 7. If there is more than one branch, the items obtained in step (6) should be arranged in alphabetical order.
• The items must be separated by: [no space]hyphen[no space]
• This rule is already known to us.
• For our present case, we get: 6-ethyl-2-methyl
Rule 8. The result obtained in (7) must be written as prefix to the name of the parent chain.
• There must be no space after the prefix.
• This rule is already known to us.
• For our present case, we get: 6-ethyl-2-methylnonane.
• This is the correct IUPAC name.
Now we will see some sub rules. Sub rules may be required while naming complex molecules.
Rule 5A. We have seen rule 5. It helps us to number the C atoms in the correct order. In some cases, the numbering may not be so simple. This can be explained using an example. It can be written in steps:
(i) Consider the molecule in fig.12.26(a) below:
 |
| Fig.12.26 |
• Applying rule 2, we see that ‘oct’ must be used.
• Applying rule 3, we get: octane
• Applying rule 4, we see that, there is a methyl group and an ethyl group. This is shown in fig.b
• Applying rule 5, we see two possibilities:
♦ In fig.c, the numbers are 3 and 6
♦ In fig.d also, the numbers are 3 and 6
(ii) One can argue that, both numbering systems are correct because, both give 3 and 6
(iii) But in such cases, we use rule 5A:
If two branches are present in equivalent positions, then the lower number should be given to that branch which comes first in the alphabetical order.
(iv) In our present case, 'ethyl' comes before 'methyl' in the alphabetical order. So the ethyl group must get the lower number 3. That means, fig.c is correct. We must discard fig.d
• This rule 5A is not known to us before.
(v) Let us go ahead and apply the remaining rules. Applying rule 6, we get:
3-ethyl
6-methyl
(vi) Applying rule 7, we get: 3-ethyl-6-methyl
(vii) Applying the final rule 8, we get the IUPAC name: 3-ethyl-6-methyloctane.
Rule 7A. This is similar to rule 7 that we saw above. After applying rule 6, we may want to apply this rule 7A instead of rule 7. It depends on the given molecule. This rule is applicable when two or more identical branches are present. It can be explained using an example. It can be written in 5 steps:
(i) Consider the molecule in fig.12.27(a) below:
 |
| Fig.12.27 |
• Applying rule 1, we see that the parent chain contains five C atoms.
• Applying rule 2, we see that ‘pent’ must be used.
• Applying rule 3, we get: pentane
• Applying rule 4, we see that, both the branches are methyl groups.
• Applying rule 5, we see that, numbering can be done from either sides. Both will give the same result: 2 and 4
• Applying rule 6, we get two items:
♦ 2-methyl
♦ 4-methyl
(ii) The branches are identical. So the alphabetical order is not applicable. That means, we cannot apply rule 7 in this case.
(iii) Here we use rule 7A.
• According to this rule:
♦ If there are two identical branches, we use the prefix ‘di’
♦ If there are three identical branches, we use the prefix ‘tri’
♦ If there are four identical branches, we use the prefix ‘tetra’
♦ If there are five identical branches, we use the prefix ‘penta’
♦ If there are six identical branches, we use the prefix ‘hexa’
• The position numbers should be written before the above prefixes.
• The position numbers should be separated by commas.
• After the position numbers, there must be: [no space]hyphen[no space]
• The correct format is:Position number,Position number-di/tri/tetra/
(iv) This rule is already known to us. So after applying rule 6, we apply rule 7A.
Rule 7A helps us to arrange the items obtained in rule 6. We get:
2,4-Dimethyl
(v) Now we can apply rule 8. We get:
2,4-Dimethylpentane
Another example: This can be written in 5 steps:
(i) Consider the molecule in fig.12.28(a) below:
 |
| Fig.12.28 |
• Applying rule 1, we see that the parent chain contains five C atoms.
• Applying rule 2, we see that ‘pent’ must be used.
• Applying rule 3, we get: pentane
• Applying rule 4, we see that, all three branches are methyl groups.
• Applying rule 5, we see that, numbering should be done from left to right
♦ Left to right will give: 2,2,4
♦ Right to left will give larger numbers: 2,4,4
• Applying rule 6, we get three items:
♦ 2-methyl
♦ 2-methyl
♦ 4-methyl
(ii) The branches are identical. So the alphabetical order is not applicable. That means, we cannot apply rule 7 in this case.
(iii) Here we use rule 7A.
• According to this rule:
♦ If there are three identical branches, we use the prefix ‘tri’
• The position numbers should be written before the above prefix.
• The position numbers should be separated by commas.
• After the position numbers, there must be: [no space]hyphen[no space]
• The correct format is: Position number,Position number-tri
(iv) This rule is already known to us. So after applying rule 6, we apply rule 7A.
Rule 7A helps us to arrange the items obtained in rule 6. We get:
2,2,4-Trimethyl
(v) Now we can apply rule 8. We get:
2,2,4-Trimethylpentane
Rule 7B. This is similar to rules 7 and 7A that we saw above. After
applying rule 6, we may want to apply this rule 7B instead of rule 7 and rule 7A. It
depends on the given molecule. This rule is applicable when two or
more identical branches of different types are present. It can be explained using an
example. It can be written in 5 steps:
(i) Consider the molecule in fig.12.29(a) below:
 |
| Fig.12.29 |
• Applying rule 1, we see that the parent chain contains seven C atoms.
• Applying rule 2, we see that ‘hept’ must be used.
• Applying rule 3, we get: heptane
• Applying rule 4, we see that, two methyl groups and one ethyl group is present.
• Applying rule 5, we see that, numbering should be done from left to right
♦ Left to right will give: 3,4,4
♦ Right to left will give larger numbers: 4,4,5
• Applying rule 6, we get three items:
♦ 3-ethyl
♦ 4-methyl
♦ 4-methyl
(ii) Two branches are identical. So the alphabetical order is not applicable. That means, we cannot apply rule 7 in this case.
(iii) We cannot use rule 7A either because, there are different types of branches.
(iv) So we use rule 7B.
• According to this rule:
We need not consider the prefixes di, tri, etc., while arranging the branches in alphabetical order.
(v) This rule is not known to us. Let us apply it to our present case:
• By applying rule 6, we get two items:
3-ethyl and 4,4-Dimethyl
• If we follow the exact alphabetical order, we must write 4,4-Dimethyl before 3-ethyl. This is because, 'D' comes before 'e' in the alphabetical order.
• But rule 7B tells us that, Di, tri, tetra etc., should not be considered for alphabetical order.
• So we must write 3-ethyl before 4,4-Dimethyl.
(vi) Now we can apply rule 8. We get:
3-Ethyl-4,4-dimethylheptane.
In the next section, we
will see how to give proper names to the branches.
Previous
Contents
Next
Copyright©2021 Higher secondary chemistry.blogspot.com
No comments:
Post a Comment