In the previous section, we saw how eight rules help us to name branched chain hydrocarbons. Recall that, the branches that we saw were small. We were able to give simple names like methyl, ethyl, propyl etc., But if there are branches within those branches, the naming will become more complicated. However, IUPAC has given specific rules that can help us in such situations. In this section, we will discuss those rules.
We can become familiar with those rules by analyzing an example. It can be written in 4 steps:
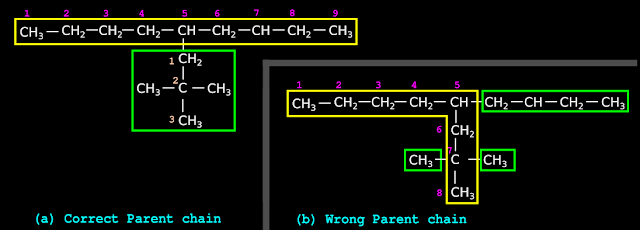
1. Consider the hydrocarbon in fig.12.30(a) below:
 |
| Fig.12.30 |
♦ The parent chain is indicated by the yellow rectangle.
♦ The branch is indicated by the green rectangle.
2. We see that, there are branches within the green rectangle.
• So first we have to give appropriate name for the green rectangle.
3. To give the name, we can use the 8 rules that we saw in the previous section. Let us apply those rules to the branch in the green rectangle.
• Applying rule 1, we see that the parent chain in the green rectangle contains four C atoms.
• Applying rule 2, we see that ‘but’ must be used.
• Applying rule 3 needs special care. Since the branch is attached to the yellow rectangle, there will be one H atom less. So we must use 'butyl' instead of 'butane'
• Applying rule 4, we see that, both the branches are methyl groups.
• Applying rule 5 needs special care. We must always start numbering from that C atom which is attached to the yellow rectangle. This is shown in fig.b. This rule is new to us.
• Applying rule 6, we get two items:
♦ 1-methyl
♦ 3-methyl
• Applying rule 7A, we get: 1,3-Dimethyl
• Applying rule 8, We get: 1,3-Dimethylbutyl.
♦ This must be written in parenthesis. We get: (1,3-Dimethylbutyl)
4. Now we can apply the eight rules to the yellow rectangle.
• Applying rule 1, we see that the parent chain contains twelve C atoms.
• Applying rule 2, we see that ‘dodec’ must be used.
• Applying rule 3, we get: dodecane
• Applying rule 4: We have already named the branch in step 3 above.
♦ We got: (1,3-Dimethylbutyl)
• Applying rule 5, we see that, numbering must be done from right. We get the number 6
• Applying rule 6, we get:
♦ 6-(1,3-Dimethylbutyl)
• Applying rule 7: There is only one branch. So there is no need to apply rule 7
• Applying rule 8, We get: 6-(1,3-Dimethylbutyl)dodecane
| ◼ The rules that we learn from the above example are: (i) The eight rules can be used to name the branch in the green rectangle. (ii) Numbering must begin from the C atom which is attached to the yellow rectangle. (iii) The final name of the branch in the green rectangle must be written in parenthesis. |
Another example can be written in 6 steps:
1. Consider the hydrocarbon in fig.12.31(a) below:
 |
| Fig.12.31 |
♦ The parent chain is indicated by the yellow rectangle.
✰ We see that the parent chain has ten C atoms.
♦ The branches are indicated by the green rectangles.
2. Fig.12.31(b) shows the same molecule as in fig.(a)
♦ The parent chain is indicated by the yellow rectangle.
✰ We see that the parent chain has ten C atoms.
♦ The branches are indicated by the green rectangles.
3. In both figs. (a) and (b), the parent chain has ten C atoms. So which one shall we choose?
• The IUPAC rules will help us to answer the question.
According to IUPAC, we must choose that parent chain which has the largest number of branches.
• This rule is new to us.
4. Let us apply it to our present case.
• We see that:
♦ In fig.12.31(a), there are two branches
♦ In fig.12.31(b), there are three branches
• So we must choose the parent chain in fig.b
5. Next step is to write the names of the branches in fig.b
• We know that, the small green rectangles are methyl groups.
• The large green rectangle requires special attention. We can write it's name using the eight rules that we saw in the previous section.
• Applying rule 1, (numbering from left), we see that the parent chain in the green rectangle contains five C atoms. But we have seen that, the numbering must begin from the C atom which is attached to the yellow rectangle. When the C atoms are numbered in this way, there will be four C atoms in the parent chain.
• Applying rule 2, we see that ‘but’ must be used.
• Applying rule 3 needs special care. Since the branch is attached to the yellow rectangle, there will be one H atom less. So we must use 'butyl' instead of 'butane'
• Applying rule 4, we see that, the branch is an ethyl group.
• Applying rule 5: We have already started numbering from that C atom which is attached to the yellow rectangle.
• Applying rule 6, we get: 2-ethyl
• Applying rule 7: There is only one branch. So there is no need to apply rule 7
• Applying rule 8, We get: 2-ethylbutyl.
♦ This must be written in parenthesis. We get: (2-ethylbutyl)
6. Now we can apply the eight rules to the yellow rectangle.
• Applying rule 1, we see that the parent chain contains ten C atoms.
• Applying rule 2, we see that ‘dec’ must be used.
• Applying rule 3, we get: decane
• Applying rule 4: We have already named the branches in step 4 above.
• Applying rule 5, we see that, numbering must be done from bottom. We get the numbers 3,3,5
♦ If it is numbered from left, we will get: 6,8,8. These are larger numbers.
• Applying rule 6, we get 3 items:
♦ 3-methyl
♦ 3-methyl
♦ 5-(2-ethylbutyl)
• Applying rule 7: This rule is not applicable because, there are identical branches.
• Applying rule 7A, the items become:
♦ 3,3-Dimethyl
♦ 5-(2-ethylbutyl)
• Applying rule 7B, we see that 'D' in 'Di' should not be considered for alphabetical listing. So 5-(2-ethylbutyl) must come first.
• Applying rule 8, we get: 5-(2-Ethylbutyl)-3,3-dimethyldecane
• We learned a new rule from this example. So the earlier table can be modified:
| ◼ The rules that we learn from the above two examples are: (i) The eight rules can be used to name the branch in the green rectangle. (ii) Numbering must begin from the C atom which is attached to the yellow rectangle. (iii) The final name of the branch in the green rectangle must be written in parenthesis. (iv) We must choose that parent chain which has the largest number of branches. |
Let us see what the name will become, if we choose the yellow rectangle in fig.(a) to be the parent branch. It can be written in 3 steps:
1. Let us write the names of the branches in fig.a
• We know that, the small green rectangle is ethyl group.
•
The large green rectangle requires special attention. We can write it's
name using the eight rules that we saw in the previous section.
•
Applying rule 1, we see that the parent chain in
the green rectangle contains four C atoms.
• Applying rule 2, we see that ‘but’ must be used.
•
Applying rule 3 needs special care. Since the branch is attached to the
yellow rectangle, there will be one H atom less. So we must use 'butyl'
instead of 'butane'
• Applying rule 4, we see that, the branches are methyl groups.
•
Applying rule 5: We have already started numbering from
that C atom which is attached to the yellow rectangle.
• Applying rule 6, we get two items:
♦ 2-methyl
♦ 2-methyl
• Applying rule 7: Two branches are identical so we cannot apply rule 7
Applying rule 7A, we get: 2,2-Dimethyl
• Applying rule 8, We get: 2,2-Dimethylbutyl.
♦ This must be written in parenthesis. We get: (2,2-Dimethylbutyl)
2. Now we can apply the eight rules to the yellow rectangle.
• Applying rule 1, we see that the parent chain contains ten C atoms.
• Applying rule 2, we see that ‘dec’ must be used.
• Applying rule 3, we get: decane
• Applying rule 4: We have already named the branches in step 1 above.
• Applying rule 5, we see that, numbering must be done from right. We get the numbers 3,5
♦ If it is numbered from left, we will get: 6,8. These are larger numbers.
• Applying rule 6, we get 2 items:
♦ 3-ethyl
♦ 5-(2,2-Dimethylbutyl)
•
Applying rule 7B, we see that 'D' in 'Di' should not be considered for
alphabetical listing. So 3-ethyl must come first.
• Applying rule 8, we get: 3-Ethyl-5-(2,2-dimethylbutyl)decane
3. Thus we obtained a name. But this name is not valid because, the parent chain that we chose was wrong.
Let us see one more example. It can be written in 5 steps:
1. Consider the hydrocarbon in fig.12.32(a) below:
 |
| Fig.12.32 |
♦ The parent chain is indicated by the yellow rectangle.
✰ We see that the parent chain has nine C atoms.
♦ The branches are indicated by the green rectangles.
✰ There is only one branch.
2. Fig.12.32(b) shows the same molecule as in fig.(a)
♦ The parent chain is indicated by the yellow rectangle.
✰ We see that the parent chain has eight C atoms.
♦ The branches are indicated by the green rectangles.
✰ There are three branches.
3. Though the number of branches is greater in fig.b, we must choose only that parent chain which has the greatest number of C atoms. So we must choose fig.a
4. Next step is to write the name of the branch in fig.a
•
We can write it's
name using the eight rules that we saw in the previous section.
•
Applying rule 1, (numbering from the C atom which is connected to the yellow rectangle), we see that the parent chain in
the green rectangle contains three C atoms.
• Applying rule 2, we see that ‘prop’ must be used.
•
Applying rule 3 needs special care. Since the branch is attached to the
yellow rectangle, there will be one H atom less. So we must use 'propyl'
instead of 'propane'
• Applying rule 4, we see that, the branches are methyl groups.
•
Applying rule 5: We have already started numbering from
that C atom which is attached to the yellow rectangle.
• Applying rule 6, we get two items:
♦ 2-methyl
♦ 2-methyl
• Applying rule 7: There are two identical branches. So we apply rule 7A. We get: 2,2-Dimethyl
• Applying rule 8, We get: 2,2-Dimethylpropyl.
♦ This must be written in parenthesis. We get: (2,2-Dimethylpropyl)
5. Now we can apply the eight rules to the yellow rectangle.
• Applying rule 1, we see that the parent chain contains nine C atoms.
• Applying rule 2, we see that ‘non’ must be used.
• Applying rule 3, we get: nonane
• Applying rule 4: We have already named the branch in step 4 above.
• Applying rule 5, we see that, numbering can be done either from left or right. We will get the same number 5
• Applying rule 6, we get: 5-(2,2-Dimethylpropyl)
• Applying rule 7: There is no need to apply this rule because, there is only one branch.
• Applying rule 8, we get: 5-(2,2-Dimethylpropyl)nonane
Now let us see some special cases. It is related to trivial names of branches. It can be written in 8 steps:
1. While naming a hydrocarbon,
♦ We first identify the parent chain (yellow rectangle).
♦ Then we identify the branches (green rectangles).
2. We have seen that, the green rectangles are alkyl groups.
• We also saw that, if branches are present inside the green rectangles, we will have to first establish the names of the green rectangles.
3. Consider the following situation. It is written in 2 steps:
(i) The green rectangle is a simple alkyl group (which has upto five C atoms).
(ii) However it contains some branches.
4. This situation arises when the alkyl groups are:
♦ propyl- ( three C atoms)
♦ butyl- ( four C atoms)
♦ pentyl- ( five C atoms)
• Let us analyze such situations.
5. Consider the case when the green rectangle contains three C atoms. This can be analyzed in 4 steps:
(i) If those three C atoms are in a straight chain, we can readily name it as propyl-
(ii) But if those three C atoms are in branched arrangements, we will have to give each of those arrangements, a distinct name.
(iii) When the number of C atoms is three, there is only one possible arrangement in which it can be branched. It is shown in fig.12.33(a) below.
(iv) Since there is only one possible arrangement, we can give it a fixed name.
• In fact, it has had a fixed name even before the IUPAC system was enforced. That means, it has a trivial name. The name is: Isopropyl-
 |
| Fig.12.33 |
6. Consider the case when the green rectangle contains four C atoms. This can be analyzed in 4 steps:
(i) If those four C atoms are in a straight chain, we can readily name it as butyl-
(ii) But if those four C atoms are in branched arrangements, we will have to give each of those arrangements, a distinct name.
(iii) When the number of C atoms is four, there are three possible arrangements in which it can be branched. They are shown in fig.12.33(b), (c) and (d) above.
(iv) Since there are only three possible arrangements, we can give each of them a fixed name.
• In fact, each of them has had a fixed name even before the IUPAC system was enforced. That means, they have trivial names. The names are: sec-Butyl-, Isobutyl- and tert-Butyl-
7. Consider the case when the green rectangle contains five C atoms. This can be analyzed in 4 steps:
(i) If those five C atoms are in a straight chain, we can readily name it as pentyl-
(ii) But if those five C atoms are in branched arrangements, we will have to give each of those arrangements, a distinct name.
(iii) When the number of C atoms is five, there are many possible arrangements in which it can be branched. The most commonly occurring arrangement is shown in fig.12.33(e).
(iv) Since it is a commonly occurring arrangement, we can give it a fixed name.
• In fact, it has had a fixed name even before the IUPAC system was enforced. That means, it has a trivial name. The name is: Neo-Pentyl-
8. IUPAC allows us to use the above five trivial names.
Let us see an example. It can be written in 4 steps:
1. Consider the hydrocarbon in fig.12.34(a) below:
 |
| Fig.12.34 |
♦ The parent chain is indicated by the yellow rectangle.
♦ The branches are indicated by the green rectangles.
2. We see that, there are branches within the green rectangles.
• So first we have to give appropriate name for the green rectangles.
3. Fortunately, both the branches in this case, have trivial names which are allowed by the IUPAC.
♦ The top branch is isopropyl
♦ The bottom branch is sec-Butyl
4. Now we can apply the eight rules to the yellow rectangle.
• Applying rule 1, we see that the parent chain contains ten C atoms.
• Applying rule 2, we see that ‘dec’ must be used.
• Applying rule 3, we get: decane
• Applying rule 4: We have already named the branches in step 3 above. We got:
♦ isopropyl
♦ sec-Butyl
• Applying rule 5, we see that, numbering must be done from left. We get the numbers 4,5
• Applying rule 6, we get:
♦ 4-isopropyl
♦ 5-sec-Butyl
• Applying rule 7: Here we have to apply rule 7B.
• For alphabetical listing, IUPAC recommends that:
♦ 'iso' and 'neo' should be considered as part of the fundamental alkyl name.
♦ 'sec' and 'tert' should not be considered so.
• This rule is new to us.
• Let us apply this to our present case:
♦ 'i' in isopropyl comes before 's' in sec-butyl
♦ So it seems that 4-isopropyl must be written before 5-sec-Butyl
♦ But 'sec' should not be considered.
♦ We must consider only 'Butyl' for alphabetical listing.
♦ So 5-sec-Butyl must be written before 4-isopropyl.
• Applying rule 8, We get: 5-sec-Butyl-4-isopropyldecane
• We learned a new rule from this example. So the earlier table can be modified:
| ◼ The five rules (in addition to the eight rules) to be considered when the green rectangles have branches inside them are: (i) The eight rules can be used to name the branch in the green rectangle. (ii) Numbering must begin from the C atom which is attached to the yellow rectangle. (iii) The final name of the branch in the green rectangle must be written in parenthesis. (iv) We must choose that parent chain which has the largest number of branches. (v) 'iso' and 'neo' should be considered as part of the fundamental alkyl name. ♦ 'sec' and 'tert' should not be considered so |
Let us see a solved example.
Solved example 12.7
Structures and IUPAC names of some hydrocarbons are given below. Explain why the names given in the parenthesis are incorrect.
Solution:
Part (a):
1. First we number the C atoms in the parent chain.
• While numbering from left to right, the branches get the numbers: 2,5,6
• While numbering from right to left, the branches get the numbers: 3,4,7
2. The lowest number 2 is obtained when the numbering is from left to right. So it is the correct numbering.
• Numbering from right to left is wrong.
• Thus we get the IUPAC name: 2,5,6-Trimethyloctane.
Part (b):
1. First we number the C atoms in the parent chain.
• While numbering from left to right, the branches get the numbers: 3,5
• While numbering from right to left, the branches get the same numbers: 3,5
2. So the branches are in equivalent positions.
• We must apply rule 5A that we saw in the previous section.
• According to the rule, the branch which comes first in the alphabetical listing, must get the lowest number.
3. In our present case, the branches are: methyl and ethyl.
• In the alphabetical listing, ethyl will come first. So it must get the lower number 3.
• Thus we get: 3-Ethyl-5-methylheptane.
In the next section, we
will see nomenclature of cyclic compounds.
Previous
Contents
Next
Copyright©2021 Higher secondary chemistry.blogspot.com

No comments:
Post a Comment