In the previous section, we completed a discussion on some basic principles and techniques in organic chemistry. In this chapter, we will see hydrocarbons.
Some basics about hydrocarbons can be written in 7 steps:
1. Consider LPG that we use commonly for cooking purposes. LPG is the abbreviated form of Liquefied Petroleum Gas.
• Let us see some basic details about LPG. It can be written in 4 steps:
(i) Crude oil is a thick black liquid that occurs naturally at many places below the earth’s crust. This oil is refined to obtain useful products like petrol, kerosene etc.,
(ii) Petroleum gas is obtained during this refining process. This gas is liquefied by applying pressure and lowering temperature.
(iii) In the liquid state, this fuel can be easily stored and transported in cylinders and tanks. Thus it becomes available to us for cooking purposes.
(iv) When LPG burns, pollution occurs only to a very small extent.
2. CNG is another fuel. It is used in automobiles. CNG is the abbreviated form of Compressed Natural Gas.
• Let us see some basic details about CNG. It can be written in 3 steps:
(i) Natural gas is a gas which forms naturally above crude oil deposits.
(ii) This gas is collected and compressed to reduce volume. It can then be transported in cylinders, tanks or through pipe lines.
(iii) Like LPG, the CNG also causes pollution only to a very small extent.
3. Other fuels like petrol, diesel and kerosene are already familiar to us.
4. All these fuels are mixtures of various hydrocarbons.
◼ Hydrocarbons are compounds containing only hydrogen and carbon.
5. We know that hydrogen can undergo combustion in the presence of oxygen.
• The balanced equation is:
2H2 (g) + O2 (g) ⟶ 2H2O(g)
6. Similarly, carbon can also undergo combustion in the presence of oxygen.
• The balanced equation is:
C (s) + O2 (g) ⟶ CO2 (g)
7. Hydrocarbons are compounds containing both hydrogen and carbon. So they are good fuels.
• For example, methane can undergo combustion in the presence of oxygen. The balanced equation is:
CH4 (g) + 2O2 (g) ⟶ CO2 (g) + 2H2O (g)
• The main components of LPG are propane and butane.
• The main component of CNG is methane.
• Petrol, diesel and kerosene contain mixtures of various hydrocarbons.
Classification of hydrocarbons
• Some basics about classification can be written in 6 steps:
1. Since there are a very large number of hydrocarbons, it is essential to classify them into various categories.
• The three main categories are:
(i) Saturated hydrocarbons
(ii) Unsaturated hydrocarbons
(iii) Aromatic hydrocarbons.
2. We know that, carbon is tetravalant. That is., it needs four electrons to attain stability.
• Those four electrons can be obtained through single bonds, double bonds or triple bonds.
3. In saturated hydrocarbons, all bonds will be single bonds.
• Saturated hydrocarbons can be further classified as alkanes and cycloalkanes.
• In alkanes, the first and last C atoms in the chain will not be bonded together. So they do not form closed chains. They are open chain hydrocarbons.
• In cycloalkanes, the first and last C atoms in the chain will be bonded together. So they form closed chains.
4. In unsaturated hydrocarbons, one or more bonds will be double or triple bonds.
• If one or more bonds are double bonds, it is classified as an alkene.
• If one or more bonds are triple bonds, it is classified as an alkyne.
• Like cycloalkanes, cycloalkenes and cycloalkynes are also possible.
5. So the classification of hydrocarbons can be represented as in fig.13.1 below:
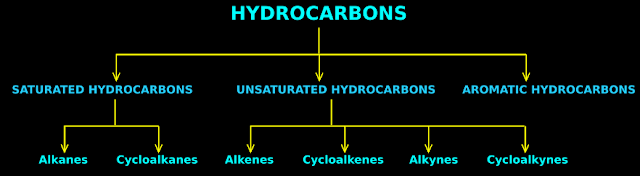 |
| Fig.13.1 |
6. Aromatic hydrocarbons are a special type of cyclic compounds. The name is derived from the fact that, they have a pleasant odour.
• They are also known as arenes.
Alkanes
• Some basics about alkanes can be written in 13 steps:
1. All bonds in alkanes are single bonds.
2. The first member of the alkane family is methane (CH4).
3. If we remove one H atom from CH4, and put a C atom in it’s place, we get the second member.
• But the valencies of the new C atom should also be satisfied with H atoms. So the second member is C2H6
4. If we remove one H atom from C2H6, and put a C atom in it’s place, we get the third member.
• But the valencies of the new C atom should also be satisfied with H atoms. So the third member is C3H8
5. In this way we can obtain a large number of members. We can write:
• Each member is obtained by two steps:
(i) Remove an H atom from the preceding member.
(ii) Put a -CH3 group in the place of that H atom.
6. We see that:
♦ Each member has one C atom more than it’s preceding member.
♦ Also each member has two H atoms more than it’s preceding member.
7. The general formula of this homologous series is: CnH2n+2
• n is the number of C atoms.
• If we know the value of n, we can easily calculate the number of H atoms.
8. The first member methane has the simplest structure among all alkanes. It is a tetrahedral structure. We saw the details in an earlier chapter. [see section 4.25]
• Fig.13.2 (a) below shows the ball and stick model of methane.
 |
| Fig.13.2 |
Some features of this model can be written in 8 steps:
(i) The central pink sphere represents the C atom.
(ii) The four orange spheres represent the four H atoms.
(iii) The sticks are painted with two different colors.
♦ The pink color indicates that, one electron in the bond belongs to C.
♦ The orange color indicates that, the other electron in the bond belongs to H.
(iv) The red arrow represents x-axis.
(v) The green arrow represents y-axis.
(vi) The C atom is situated at the origin.
(vii) Consider the stick between the top H atom and the C atom. This stick is perpendicular to the xy-plane.
(viii) If we rotate this CH4 molecule through 90o about the y-axis, the top H atom and it's stick will become aligned with the x-axis.
• This rotation is indicated by the yellow curved arrow.
• The resulting orientation obtained after rotation, is shown in fig.b. It is a rotated tetrahedral structure.
9. Now we can write about the structure of the second member ethane (C2H6). It can be written in 4 steps:
(i) Consider the orientation of tetrahedral CH4 molecule in fig.13.2(b) above.
• Two such tetrahedra are lying along the x-axis and are facing each other in fig.13.3(a) below:
 |
| Fig.13.3 |
(ii) Consider the tetrahedron on the left side. One of it's H atoms lies on the x-axis. This H atom is removed. It then becomes -CH3
(iii) Consider the tetrahedron on the right side. One of it's H atoms lies on the x-axis. This H atom is removed. It then becomes -CH3
(iv) Removal of H atoms will make the molecules unstable. But the two -CH3 can combine together by making a direct bond between the two C atoms. This is shown in fig.18.3(b). It is a C2H6 molecule.
• We can write:
The ethane molecule contains two tetrahedral structures.
10. Now we can write about the structure of the third member propane (C3H8). It can be written in 5 steps:
(i) Consider the orientation of tetrahedral CH4 molecule in fig.13.2(b) above.
• Two such tetrahedra are lying along the x-axis and are facing each other in fig.13.4(a) below. Also a third tetrahedron is approaching from the +ve side of the y-axis.
 |
| Fig.13.4 |
(ii) Consider the tetrahedron on the left side. One of it's H atoms lies on the x-axis. This H atom is removed. It then becomes -CH3
(iii) Consider the tetrahedron on the right side. One of it's H atoms lies on the x-axis. This H atom is removed. It then becomes -CH3
(iv) Consider the third tetrahedron which approaches from the +ve side of the y-axis. Two of it's H atoms are removed. It then becomes -CH2
(iv) Removal of H atoms will make the molecules unstable. But the two -CH3 can combine with the -CH2 by making direct bonds between the three C atoms. This is shown in fig.18.4(b). It is a C3H8 molecule.
• We can write:
The propane molecule contains three tetrahedral structures.
(v) It may be noted that, the three C atoms do not fall along a line.
11. In this way, tetrahedra are joined together to form members of the alkane series.
• The 3D model of butane is shown in fig.13.5 below.
• We can see that:
♦ One -CH3 tetrahedron is present at each end of the chain.
♦ Two -CH2 tetrahedra are present in the middle.
 |
| Fig.13.5 By Ben Mills and Jynto The original file can be seen here. |
12. Scientists have determined the bond lengths in alkanes,
♦ The C-C bond length is 154 pm
♦ The C-H bond length is 112 pm
13. It is important to remember that, in alkanes, there are no π bonds.
♦ All C-C bonds are 𝜎 bonds.
♦ All C-H bonds are 𝜎 bonds.
• We saw those details in the previous chapter.
In the next section we will see nomenclature of alkanes.
Copyright©2022 Higher secondary chemistry.blogspot.com
No comments:
Post a Comment