In the previous section, we completed a discussion on the nomenclature and isomerism in alkanes. In this section, we will see preparation of alkanes.
We have to learn three methods used for preparing alkanes.
I. Preparation of alkanes from unsaturated hydrocarbons.
This can be written in 6 steps:
1. We know that unsaturated hydrocarbons contain double or triple bonds.
• If enough dihydrogen is supplied, those double or triple bonds can be converted into single bonds. Thus we can prepare alkanes. This process is known as hydrogenation.
2. This process requires the presence of finely divided catalysts like platinum, palladium or nickel.
• If platinum or palladium is used, room temperature will be sufficient to carry out the reaction.
• If nickel is used, high temperature and pressure will be required.
3. When catalysts are present, the H-H bond in dihydrogen breaks.
• The H atoms get absorbed on the surface of the catalysts.
• These H atoms are then transferred to the unsaturated hydrocarbons.
4. The chemical equation for the hydrogenation of ethene is:
$\rm{CH_2 = CH_2~+~H_2~ \color {green}{\xrightarrow[{}]{Pt/Pd/Ni}} ~ CH_3 - CH_3}$
5. The chemical equation for the hydrogenation of propene is:
$\rm{CH_3 - CH = CH_2~+~H_2~ \color {green}{\xrightarrow[{}]{Pt/Pd/Ni}} ~ CH_3 - CH_2 - CH_3}$
6. The chemical equation for the hydrogenation of propyne is:
$\rm{CH_3 - C ≡ CH~+~2H_2~ \color {green}{\xrightarrow[{}]{Pt/Pd/Ni}} ~ CH_3 - CH_2 - CH_3}$
II. Preparation of alkanes from alkyl halides
Method A:
This can be written in 4 steps:
1. Alkyl halides can be reduced using zinc and dilute hydrochloric acid to give alkanes.
• As an example, we can write the chemical equation for the reduction of chloromethane:
$\rm{CH_3 - Cl~+~H_2~ \color {green}{\xrightarrow[{}]{Zn,\;H^+}} ~ CH_4~+~HCl}$
• It is clear that the C-Cl bond in chloromethane undergoes fission. We have seen the mechanism of this fission in the previous chapter. [see fig.12.68 of section 12.10]
• We will see more details about this type of reactions in later sections.
2. Note that, in this method, we are dealing with alkyl halides.
Halogens are: F, Cl, Br and I
• But for the preparation of alkanes by this method, we cannot use alkyl fluorides. Only chlorides, bromides and iodides can be used. This is because, F being highly electronegative, the C-F bond will be very strong and hence difficult to break.
3. The chemical equation for the reduction of chloroethane is:
$\rm{CH_3 - CH_2 - Cl~+~H_2~ \color {green}{\xrightarrow[{}]{Zn,\;H^+}} ~ CH_3 - CH_3~+~HCl}$
4. The chemical equation for the reduction of 1-chloropropane is:
$\rm{CH_3 - CH_2 - CH_2 - Cl~+~H_2~ \color {green}{\xrightarrow[{}]{Zn,\;H^+}} ~ CH_3 - CH_2 - CH_3~+~HCl}$
Method B:
This can be written in 4 steps:
1. Alkyl halides can be treated with sodium metal in dry ether solution to give higher alkanes. (The ether solution should be dry because, if moisture is present, the sodium metal will react with that moisture)
• This reaction is known as Wurtz reaction.
• As an example, we can write the chemical equation for the reaction between bromomethane and sodium metal:
$\rm{CH_3 - Br~+~2Na~+~Br - CH_3~ \color {green}{\xrightarrow[{}]{dry~ether}} ~ CH_3 - CH_3~+~2NaBr}$
2. Let us write the chemical equation when bromoethane is used:
$\rm{CH_3 - CH_2 - Br~+~2Na~+~Br - CH_2 - CH_3~ \color {green}{\xrightarrow[{}]{dry~ether}} ~ CH_3 - CH_2 - CH_2 - CH_3~+~2NaBr}$
3. In both the examples that we have seen, we have used the same type of alkyl halide.
♦ In the first example, we used bromomethane only.
♦ In the second example, we used bromoethane only.
◼ Now the question arises:
What will happen if we take different alkyl halides?
• The answer can be written in 5 steps:
(i) Suppose that, we take two different alkyl halides, say CH3Br and CH3CH2Br
Then we will get three different higher alkanes as explained below:
(ii) Two molecules of CH3Br will react with two sodium atoms to give one molecule of CH3-CH3.
• This is the same reaction that we wrote in step (1)
(iii) Two molecules of CH3-CH2-Br will react with two sodium atoms to give one molecule of CH3-CH2-CH2-CH3.
• This is the same reaction that we wrote in step (2)
(iv) One molecule of CH3Br and one molecule of CH3-CH2-Br will react with two sodium atoms to give one molecule of CH3-CH2-CH3.
• The equation is:
$\rm{CH_3 - Br~+~2Na~+~Br - CH_2 - CH_3~ \color
{green}{\xrightarrow[{}]{dry~ether}} ~ CH_3 - CH_2 -
CH_3~+~2NaBr}$
(v) So from (ii), (iii) and (iv), it is clear that, the product mixture will contain three different alkanes.
• The boiling points of these alkanes are close to each other. So it will be difficult to separate them.
• So we never take different alkyl halides as reactants in Wurtz reaction.
4. We just saw that, the same type of alkyl halide is taken in Wurtz reaction. Based on this information, we can obtain an interesting result. It can be written in steps:
(i) If the alkyl halide taken has an even number of C atoms, then the product alkane will also have an even number of C atoms.
• This is because, an even number added to another even number will always give an even number.
(ii) If the alkyl halide taken has an odd number of C atoms, then also, the product alkane will have an even number of C atoms.
• This is because, an odd number added to another odd number will always give an even number.
(iii) So we can write:
Wurtz reaction can be used to produce higher alkanes having even number of C atoms.
III. Preparation of alkanes from carboxylic acids
Method A:
This can be written in 2 steps:
1. Sodium salt of a suitable carboxylic acid is heated with soda lime (soda lime is a mixture of NaOH and CaO).
• During this reaction, one C atom, two O atoms and the Na atom is removed from the salt molecule.
• Thus the salt molecule gets converted to an alkane molecule.
2. Since one C atom and two O atoms are removed, we can say that, one carbon dioxide molecule is removed.
• This process of elimination of carbon dioxide from carboxylic acid is known as decarboxylation.
• As an example, we can write the chemical equation when sodium ethanoate (the sodium salt of ethanoic acid) is heated:
$\rm{CH_3 COO^- Na^+~+~NaOH~ \color
{green}{\xrightarrow[{△}]{CaO}} ~ CH_4~+~Na_2 CO_3}$
Solved example 13.6
Sodium salt of which acid will be needed for the preparation of propane? Write the chemical equation of the reaction.
Solution:
1. During decarboxylation, one C atom is removed from the carboxylic acid.
• So we must consider that acid which has one C atom more than the number of C atoms in propane.
• That means, we must consider butanoic acid.
2. We can write:
Sodium salt of butanoic acid is needed for the preparation of propane.
3. The chemical equation is:
$\rm{CH_3 - CH_2 - CH_2 COO^- Na^+~+~NaOH~ \color
{green}{\xrightarrow[{△}]{CaO}} ~ CH_3 - CH_2 - CH_3~+~Na_2 CO_3}$
Method B:
This can be written in 2 steps:
1. In an aqueous solution, the sodium salt of the carboxylic acid will dissociate as shown below:
$\rm{CH_3 COO^- Na^+~ \rightleftarrows ~ CH_3 COO^- ~+~Na^+}$
• $\rm{CH_3 COO^- }$ is acetate ion.
• This dissociation process can be represented using Lewis structures as shown in fig.13.26 below:
 |
| Fig.13.26 |
• If this aqueous solution is subjected to electrolysis, the acetate ion will move towards the anode.
2. Consider the Lewis structure of acetate ion.
• We see that, all atoms have octet.
• But for oxygen, only the six brown electrons are original. The blue electron is acquired from the Na atom.
• So the species as a whole acquire a -ve charge. It is called the acetate ion
3. This acetate ion moves towards the anode.
• At the anode, it donates one of the brown electrons possessed by the right side O atom. The result is shown in fig.13.27(a) below:
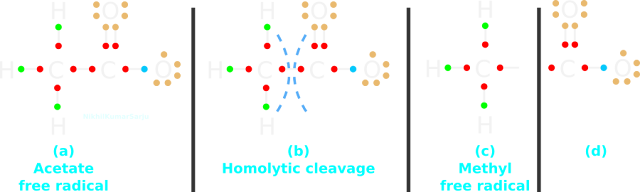 |
| Fig.13.27 |
4. So the acetate ion has lost the -ve charge. It is no longer an ion.
• But an unpaired electron is created. Thus the acetate ion has become acetate free radical.
• We have seen homolytic cleavage and formation of free radicals in the previous section [see fig.12.74 of section 12.11]
5. This free radical undergoes homolytic cleavage as shown in fig.13.27(b) above.
• As a result of this cleavage, two species are formed.
♦ The species on the left side is methyl free radical. It is shown in fig.c
♦ The species on the right side is shown in fig.d
6. So starting with an acetate ion, we obtained a methyl radical.
• A large number of acetate ions will be reaching the anode. Each of them will give a methyl free radical.
• We can group them into pairs. So each group will contain two methyl free radicals.
• Those two radicals can form a bond using the lone electrons. This will result in an ethane molecule. It is shown in fig.13.28(a) below:
 |
| Fig.13.28 |
7. We saw that, after the homolytic cleavage there will be two species.
• We saw that, the left side species (fig.13.27.a) will help to obtain ethane. Now we will see the right side species (fig.13.27.b).
• If we examine this species carefully, we can see that, the lone electrons can rearrange to form a double bond. This will result in a CO2 molecule.
• This is shown in fig.13.28(b) above.
8. The above steps help us to understand the processes taking place at the anode. Those processes can be written in a condensed form as shown in fig.13.29 below:
 |
| Fig.13.29 |
9. Next we will see the reactions taking place at the cathode. It can be written in steps:
(i) Consider the Lewis structure of water molecule. It is shown in fig.13.30(a) below:
 |
| Fig.13.30 |
• If this water molecule is near the cathode, it can receive an electron from the cathode.
• Then one of the O-H bonds will undergo homolytic cleavage. This is shown in fig.b
(ii) The left portion is a hydrogen free radical. It is very unstable. It is shown in fig.c
(iii) The right portion is also unstable. It is shown in fig.d
(iv) But the right portion obtains one electron from the cathode. Thus it becomes the hydroxyl ion. It is shown in fig.e
• Note that in fig.e, the O atom has only the six brown electrons originally. The red electron is received from some other source. In our present case, it is received from the cathode. So the structure as a whole has a negative charge.
(v) So starting with a water molecule, we obtained a hydrogen free radical.
• A large number of water molecules will be present in the solution. Each of them will give a hydrogen radical.
• We can group them into pairs. So each group will contain two hydrogen free radicals.
•
Those two radicals can form a bond using the lone electrons. This will
result in a dihydrogen molecule.
(vi) The above 5 steps help us to understand the processes taking place at the cathode. Those processes can be written in a condensed form as shown below:
At cathode:
2H2O + 2e- ⟶ 2OH- + 2 H•
H• + H• ⟶ H2 ↑
10. This method is known as Kolbe's electrolytic method.
11. This method cannot be used to prepare methane. The reason an be written in 3 steps:
(i) In fig.13.28(a), we see that, two identical alkyl free radicals combine together to form an alkane molecule.
(ii) Each of the alkyl free radicals will contain at least one C atom. So the resulting alkane will obviously contain more than one C atom.
(iii) Methane has only one C atom. So methane cannot be prepared using this method.
12. This method for preparation will always give alkanes having even number of C atoms. The reason can be written in 4 steps:
(i) In fig.13.28(a), we see that, two identical alkyl free radicals combine together to form an alkane molecule.
(ii) If the free radical has an even number of C atoms, then the product alkane will also have an even number of C atoms.
• This is because, an even number added to another even number will always give an even number.
(iii) If the free radical taken has an odd number of C atoms, then also, the product alkane will have an even number of C atoms.
• This is because, an odd number added to another odd number will always give an even number.
(iv) So we can write:
Kolbe's electrolytic method will always give alkanes having even number of C atoms.
• We have completed a discussion on the various methods for preparation of Alkanes.
• Those methods can be written in the form of a flow chart as shown in fig.13.31 below:
 |
| Fig.13.31 |
In the next section we will see physical properties of alkanes.
Copyright©2022 Higher secondary chemistry.blogspot.com
No comments:
Post a Comment