In the previous section,
we saw
the basics about resonance in organic molecules. In this
section, we will see how to compare various resonance structures of a molecule.
Rule 1:
♦ The resonance structure which has more number of covalent bonds
♦ is more stable than
♦ The resonance structure which has lesser number of covalent bonds.
•
For calculating the number of covalent bonds, a double bond is
considered as two bonds. A triple bond is considered as three bonds.
• An example is shown in fig.12.92 below.
 |
| Fig.12.92 |
• This can be explained in 4 steps:
(i) I and II are resonance structures of the same molecule.
• In I, the number of bonds is four. But in II, the number of bonds is three.
• So I will be more stable than II
(ii) Consequently, the hybrid structure will have a greater resemblance to I than II.
• We can say:
Structure I will contribute more towards the hybrid structure.
(iii) Let us check the formal charges. We have seen how to calculate formal charges in section 4.4.
We know that, any independent O atom will have six valence electrons.
• In I, we see that, the bottom O atom has gained an electron.
♦ That means, O has gained a -ve charge.
♦ Thus the formal charge of that O is ‘-’.
• In II, we see that, both top O and bottom O has gained an electron each.
♦ That means, those O atoms have gained a -ve charge each.
♦ Thus the formal charge of each of those O atoms is ‘-’.
(iv) We know that, any independent C atom will have four valence electrons.
• In II, we see that, the C atom has lost an electron.
♦ That means, C has gained a +ve charge.
♦ Thus the formal charge of that C is ‘+’.
Rule 2:
♦ The resonance structure in which all atoms have octet
♦ is more stable than
♦ The resonance structure in which one or more atoms have incomplete octet.
• An example is shown in fig.12.93 below.
 |
| Fig.12.93 |
This can be explained in 3 steps:
(i) I and II are resonance structures of the same molecule.
• In I, all atoms have octet.
• In II, the C atom has only six electrons around it.
• So I will be more stable than II
(ii) Consequently, the hybrid structure will have a greater resemblance to I than II.
• We can say:
Structure I will contribute more towards the hybrid structure.
(iii) We know that, any independent C atom will have four valence electrons.
• In II, we see that, the C has lost one of it’s electrons.
♦ That means, C has gained a +ve charge.
♦ Thus the formal charge of that C is ‘+’.
Rule 3:
♦ The resonance structure which has the least 'number of formal charges'
♦ is more stable than
♦ The resonance structure which has greater 'number of formal charges'.
• An example is shown in fig.12.94 below:
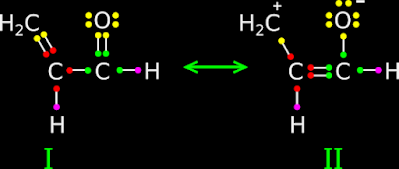 |
| Fig.12.94 |
This can be explained in 4 steps:
(i) I and II are resonance structures of the same molecule.
• In II, there are two formal charges, while in I, there are none.
• So I will be more stable than II
(ii) Consequently, the hybrid structure will have a greater resemblance to I than II.
• We can say:
Structure I will contribute more towards the hybrid structure.
(iii)
We know that, any independent C atom will have four valence electrons.
• In II, we
see that, the C atom of methyl branch has lost one of it’s electrons.
♦ That means, C has gained
a +ve charge.
♦ Thus the formal charge of that C is ‘+’.
(iv)
We know that, any independent O atom will have six valence electrons.
• In II, we
see that, the O atom has gained one electron.
♦ That means, O has gained
a -ve charge.
♦ Thus the formal charge of that O is ‘-’.
Rule 4:
♦ The resonance structure which has the -ve charge on more electronegative atom
♦ is more stable than
♦ The resonance structure which has the -ve charge on less electronegative atom.
• An example is shown in fig.12.95 below:
 |
| Fig.12.95 |
This can be explained in 4 steps:
(i) I and II are resonance structures of the same molecule.
• In II, the -ve charge is at the O atom. This is acceptable.
• In I, the -ve charge is at a C atom. This is also acceptable.
• But the -ve charge tends to be at a more
electronegative atom. When O and C are compared, O is more
electronegative.
• So II will be more stable than I
(ii) Consequently, the hybrid structure will have a greater resemblance to II than I.
• We can say:
Structure II will contribute more towards the hybrid structure.
(iii)
We know that, any independent C atom will have four valence electrons.
• In I, we
see that, the C atom of CH2 group has gained an electron.
♦ That means, C has gained
a -ve charge.
♦ Thus the formal charge of that C is ‘+’.
(iv)
We know that, any independent O atom will have six valence electrons.
• In II, we
see that, the O atom has gained one electron.
♦ That means, O has gained
a -ve charge.
♦ Thus the formal charge of that O is ‘-’.
Rule 5:
This is the reverse of rule 3.
♦ The resonance structure which has the +ve charge on more electropositive atom
♦ is more stable than
♦ The resonance structure which has the +ve charge on less electropositive atom.
Rule 6:
♦ The resonance structure in which 'distance between charges' is lesser
♦ is more stable than
♦ The resonance structure in which 'distance between charges' is greater.
• An example is shown in fig.12.96 below:
 | |
| Fig.12.96 |
• This can be explained in 3 steps:
(i) I and II are resonance structures of the same molecule.
• In I, the charges are close together (on adjacent atoms).
•
In II, the distance between the charges is larger.
• So I will be more stable than II
(ii) Consequently, the hybrid structure will have a greater resemblance to I than II.
• We can say:
Structure I will contribute more towards the hybrid structure.
(iii)
We know that, any independent C atom will have four valence electrons.
• In I, we
see that, the first C atom from left has lost an electron.
♦ That means, C has gained
a +ve charge.
♦ Thus the formal charge of that C is ‘+’.
• In I, we
see that, the second C atom from left has gained an electron.
♦ That means, C has gained
a -ve charge.
♦ Thus the formal charge of that C is ‘-’.
• In II, we
see that, the first C atom from left has lost an electron.
♦ That means, C has gained
a +ve charge.
♦ Thus the formal charge of that C is ‘+’.
• In II, we
see that, the last C atom from left has gained an electron.
♦ That means, C has gained
a -ve charge.
♦ Thus the formal charge of that C is ‘-’.
Rule 7:
Resonance structures which are equivalent, will have the same stability.
• An example is shown in fig.12.97 below:
 |
| Fig.12.97 |
• This can be explained in 3 steps:
(i) I and II are resonance structures of the same molecule.
If we rotate I about an axis passing through the CㅡH bond, we will get II.
(ii) We can apply any of the six rules written above. We will see that both are equivalent.
(iii)
We know that, any independent O atom will have six valence electrons.
• In I, we
see that, the bottom O atom has gained an electron.
♦ That means, O has gained
a -ve charge.
♦ Thus the formal charge of that O is ‘-’.
• In II, we
see that, the top O atom has gained an electron.
♦ That means, O has gained
a -ve charge.
♦ Thus the formal charge of that O is ‘-’.
Solved example 12.18
Write the resonance structures of CH2=CHㅡCHO. Indicate the relative stability of the contributing structures.
Solution:
1. The three resonance structures are shown in fig.12.98 below:
 |
| Fig.12.98 |
2. First we apply rule 1:
(a) The number of bonds in I is 9
(b) The number of bonds in II is 8
(c) The number of bonds in III is 8
(d) Based on the number of bonds:
♦ I is more stable than II
♦ I is more stable than III
• So I is the most stable among the three.
3. Based on the number of bonds, II and III has the same stability.
• So we have to apply the other rules to find which one among II and III is more stable.
4. Applying rule 2, we get:
(a) In II, the first C (from left) has an incomplete octet. All other atoms have octet.
(b) In III, the O has an incomplete octet. All other atoms have octet.
(c) So applying rule 2, both II and III have the same stability.
• We have to apply the other rules.
5. Applying rule 3, we get:
(a) The number of formal charges in II is 2
(b) The number of formal charges in III is also 2
(c) So applying rule 3, both II and III have the same stability.
• We have to apply the other rules.
6. Applying rule 4, we get:
(a) In II, the -ve charge is on the more electronegative atom, which is O
(b) In III, the -ve charge is on the lesser electronegative atom, which is C
(c) So applying rule 4, II is more stable than III
7. So the order of stability in the decreasing order is: I > II > III
• I makes the greatest contribution towards the hybrid structure.
• III makes the least contribution towards the hybrid structure.
• The hybrid structure will have more resemblance to I
Solved example 12.19
Explain why the following two structures I and II cannot be the major contributors to the real structure of CH3COOCH3
 |
| Fig.12.99 |
Solution:
1. In this problem, we are not asked to compare the two given structures. We are asked why both are not major contributors.
2. Consider I. The middle C atom do not have octet. So this structure cannot be a major contributor.
3. Consider II. A +ve charge is present on the lower O atom. This will make the structure very unstable because, O is highly electronegative. So this structure also cannot be a major contributor.
Solved example 12.20
Indicate the relative stability of the following resonance structures:
 |
| Fig.12.100 |
1. First we apply rule 1:
(a) The number of bonds in I is 6
(b) The number of bonds in II is 5
(c) The number of bonds in III is 6
(d) Based on the number of bonds:
♦ I and III are more stable than II
• So the least stable structure is II.
2. Based on the number of bonds, I and III has the same stability.
• So we have to apply the other rules to find which one among I and III is more stable.
3. Applying rule 2, we get:
(a) In I, all atoms have octet.
(b) In III also, all atoms have octet.
(c) So applying rule 2, both I and III have the same stability.
• We have to apply the other rules.
4. Applying rule 3, we get:
(a) The number of formal charges in I is 2
(b) The number of formal charges in III is also 2
(c) So applying rule 3, both II and III have the same stability.
• We have to apply the other rules.
5. Applying rule 4, we get:
(a) In I, the -ve charge is on the more electronegative atom, which is N
(b) In III, the -ve charge is on the lesser electronegative atom, which is C
(c) So applying rule 4, I is more stable than III
6. So the order of stability in the decreasing order is: I > III > II
• I makes the greatest contribution towards the hybrid structure.
• II makes the least contribution towards the hybrid structure.
• The hybrid structure will have more resemblance to I.
In the next section, we will see resonance effect.
Copyright©2021 Higher secondary chemistry.blogspot.com
No comments:
Post a Comment