• We are discussing the structure of those molecules in which hybridization in the central atom involves d-orbitals also. In the previous section 4.30, we saw the structure of PCl5. In this section, we will see SF6
Structure of SF6
• When we discussed VSEPR theory, we saw the Lewis dot structure of SF6
♦ See fig.4.98(c) in section 4.16
♦ All the five S-F bonds are single bonds
• For convenience, that fig. is shown again below:
• The details about the shape of SF6 can be written in 11 steps:
1. The S atom in SCl6 is sp3d2 hybridized
• Let us see how this sp3d2 hybridization is achieved. It can be written in 2 steps:
(i) Fig.4.181(a) below shows the orbitals of S (Sulfur)
(ii) When enough energy is given,
♦ one electron in the 3s orbital jumps to the 3di orbital
♦ one electron in the 3px orbital jumps to the 3dii orbital
• Thus we get six half filled orbitals. This is shown in fig.b
(iii) The 3s orbital mixes together with 3px, 3py, 3pz, 3di and 3dii. This is also shown in fig.b
(iv) Since there is one s-orbital, three p-orbitals, and two d orbitals, it is a sp3d2 hybridization
2. We know that:
♦ In sp3d2 hybridization, there will be six resulting hybrid orbitals
• Together, they form a square bipyramidal (octahedral) shape
♦ This can be explained in 3 steps:
(i) Fig.4.182(a) below, shows the outlines of a octahedron
♦ The base square is shown in blue dashed lines
✰ The small red sphere is the nucleus of the S atom
✰ This nucleus is situated a the 'center of gravity' of the base square
♦ Four green dashed lines radiate out from the nucleus
✰ These lines are at an angular distance of 90o apart
♦ Two red dashed lines radiate upwards and downwards from the nucleus
✰ These lines together form the axis of the octahedron
♦ The magenta dashed lines are the sloping edges of the octahedron
(ii) Lines which are required:
♦ We require the following lines:
✰ The four green dashed lines
✰ The two red dashed lines (these two, together form the axis)
♦ We do not require the following lines:
✰ The eight magenta dashed lines
✰ The four blue dashed lines
(iii) The required lines are shown in fig.4.182(b)
♦ Altogether, there are six lines
♦ The six hybrid orbitals will be oriented along these lines
♦ This is shown in fig.c
■ Thus we get the 'sp3d2 hybridized S atom'
3. Let us write three 'important points to remember' about fig.4.182:
(i) The 'plane of the base square' is exactly at the middle of the structure
♦ So this plane is called 'equatorial plane'
(ii) All the four green dashed lines lie on the 'plane of the base square'
♦ So the four orbitals along the green lines are called 'equatorial orbitals'
♦ These orbitals are at an angular distance of 90o apart
(iii) The two red dashed lines form the axis of the structure
♦ So the two orbitals along the red lines are called 'axial orbitals'
♦ They are at an angular distance of 180o apart
4. So we can write:
♦ The 'sp3d2 hybridized S atom' consists of two items:
✰ The six hybrid orbitals
✰ The nucleus (small red sphere)
• In the fig.4.178(c), the 'smaller lobes of hybrid orbitals' are not shown
♦ This is to reduce congestion and thus obtain greater clarity
5. Distribution of electrons in the hybrid orbitals:
• This can be written in 3 steps:
(i) We know that, the sp3d2 hybrid orbitals are formed from 'one s orbital', 'three p orbitals' and 'two d orbitals'
(ii) In our present case of S atom, they are: 'one 3s orbital', 'three 3p orbitals' and 'two 3d orbitals'
(iii) Before the hybridization, the orbitals mentioned in (ii) carry a total of six electrons
♦ After hybridization, the orbitals mentioned in (ii) will no longer exist
♦ Then what will happen to the six electrons?
Answer:
• The six electrons will be distributed among the six sp3d2 hybrid orbitals
♦ So each hybrid orbital will carry one electron
♦ This is indicated by the arrows in fig.4.182(c) above
• Now bonding with Cl atoms can begin
• First we will write some basic details about the Cl atom. It can be written in 2 steps:
(We have already seen the two steps in the previous section and in the previous case of PCl5. But we will write them again)
(i) The electronic configuration of Cl is: [Ne]3s23p5
• Expanding the valence orbitals, the configuration becomes: [Ne]3s2 3px2 3py2 3pz1
(ii) It is clear that, the 3pz of Cl is half filled
• This 3pz needs one more electron
• We saw it in fig.4.166 in the previous section. It is shown again below:
• The px orbital is shown in red color
♦ It has two arrows
• The py orbital is shown in green color
♦ It has two arrows
• The pz orbital is shown in blue color
♦ It has only one arrow
• The 3s orbital is represented by the cyan sphere
♦ It has two arrows
7. One Cl atom will come and overlap with each of the six half filled orbitals of the S atom
• So a total of six Cl atoms will be coming
♦ In each of those Cl atoms, it is the pz which enters into bonding
♦ This is shown in fig.4.183(a) below:
• In the fig.4.183(a) above, the 'pz orbitals of Cl' have become completely filled
8. The electron clouds of Cl:
(i) The red, green, blue and cyan regions around the Cl nucleus are mere electron clouds
♦ To define the shape of a molecule:
✰ We do not consider the positions of electrons or electron clouds
✰ We consider only the positions of 'nuclei of atoms' in that molecule
(ii) So we do not need to show the orbitals (electron clouds) of the Cl atoms. We can delete them
• However, we will retain the pz orbital. This will convey the fact that, it is 'a p orbital of Cl' that bonds with the S atom
(iii) The final structure is shown in fig.4.183(b) above
• The small yellow spheres represent the nuclei of Cl atoms
9. Thus we get the model of the SF6 molecule
• Let us write the salient features of this model. It can be written in 3 steps:
(i) There is a total of six S-F bonds
♦ Four of them lie on the equatorial plane
✰ Along the diagonals of the base square
♦ The remaining two lie along the 'axis of the octahedron'
(ii) So there are:
♦ Four equatorial bonds
♦ Two axial bonds
(iii) The angle between
♦ Any two equatorial bonds is 90o
♦ The two axial bonds is 180o
♦ Any equatorial bond and axial bond is 90o
10. The 2D representation is shown below:
• Recall that, we saw the same 2D representation when we analysed SF6 using VSEPR theory. See fig.4.98(c) in section 4.16
• There we saw the reason for giving the solid triangle, dashed triangle etc.,
11. Note that, all the bonds in SF6 are sigma bonds
• We have seen examples for sp3, sp2, sp, sp3d and sp3d2 hybridization
• Let us now write the salient features of hybridization
• We have already seen the application of those features in the various examples
• However, it is better to prepare a compilation of the features
• There are four salient features
1. The 'number of hybrid orbitals' is equal to the 'number of atomic orbitals that get hybridized'
Application example:
• In sp3d hybridization, five atomic orbitals are involved:
♦ One s orbital
♦ Three p orbitals
♦ One d orbital
• So the total number of participating orbitals is five
• After the hybridization, we get five hybrid orbitals
2. The hybrid orbitals are always equivalent in energy and shape
Application example:
• Regarding shape:
♦ In all the examples, we see the same hybrid orbitals
✰ Each of those orbitals has a larger lobe and a smaller lobe
• Regarding energy:
♦ In CH4, four hybrid orbitals are produced
✰ All four have the same energy
♦ In PCl5, five hybrid orbitals are produced
✰ All five have the same energy
3. The hybrid orbitals are more effective in forming stable bonds than pure atomic orbitals
Application example:
• Nitrogen has three half filled pure orbitals
♦ So it can form NH3
♦ But it prefers to use hybrid orbitals
♦ See section 4.28
4. The hybrid orbitals push each other as far away as possible
♦ This is to reduce repulsion
♦ So they orient in some specific directions
♦ Since there are specific directions, we are able to find the shapes of various molecules
Application example:
• As a result of hybridization in CH4, four hybrid orbitals are produced
♦ Each of those hybrid orbitals try to push the other as far away as possible
♦ This ‘pushing’ results in a tetrahedral shape
♦ Thus the shape of CH4 is tetrahedral
1. The orbitals present in the valence shell of the atom are hybridized
Application:
• We need not consider the inner orbitals while analyzing hybridization in an atom
• We need to consider only the valence shell orbitals
2. The orbitals undergoing hybridization should have almost equal energy
Application example:
• In PCl5, we saw that 4s, 3p and 3d of P cannot hybridize together
♦ This is because, their energies differ vastly
♦ See fig.4.176 of the previous section
3. Promotion of electron is not an essential condition prior to hybridization
Application example:
• In PCl5, we saw that, one electron in the 3s orbital gets promoted to a 3d orbital
♦ This promotion may take place even after the formation of the five hybrid orbitals
4. It is not necessary that, only half filled orbitals participate in hybridization. In some cases, even filled orbitals in the valence shell take part in hybridization
Application example:
• In NH3, the 2s orbital is completely filled
♦ Even then it participates in hybridization
♦ See fig.4.160 in section 4.28
Structure of SF6
• When we discussed VSEPR theory, we saw the Lewis dot structure of SF6
♦ See fig.4.98(c) in section 4.16
♦ All the five S-F bonds are single bonds
• For convenience, that fig. is shown again below:
• The details about the shape of SF6 can be written in 11 steps:
1. The S atom in SCl6 is sp3d2 hybridized
• Let us see how this sp3d2 hybridization is achieved. It can be written in 2 steps:
(i) Fig.4.181(a) below shows the orbitals of S (Sulfur)
 |
| Fig.4.181 |
♦ one electron in the 3s orbital jumps to the 3di orbital
♦ one electron in the 3px orbital jumps to the 3dii orbital
• Thus we get six half filled orbitals. This is shown in fig.b
(iii) The 3s orbital mixes together with 3px, 3py, 3pz, 3di and 3dii. This is also shown in fig.b
(iv) Since there is one s-orbital, three p-orbitals, and two d orbitals, it is a sp3d2 hybridization
2. We know that:
♦ In sp3d2 hybridization, there will be six resulting hybrid orbitals
• Together, they form a square bipyramidal (octahedral) shape
♦ This can be explained in 3 steps:
(i) Fig.4.182(a) below, shows the outlines of a octahedron
 |
| Fig.4.182 |
✰ The small red sphere is the nucleus of the S atom
✰ This nucleus is situated a the 'center of gravity' of the base square
♦ Four green dashed lines radiate out from the nucleus
✰ These lines are at an angular distance of 90o apart
♦ Two red dashed lines radiate upwards and downwards from the nucleus
✰ These lines together form the axis of the octahedron
♦ The magenta dashed lines are the sloping edges of the octahedron
(ii) Lines which are required:
♦ We require the following lines:
✰ The four green dashed lines
✰ The two red dashed lines (these two, together form the axis)
♦ We do not require the following lines:
✰ The eight magenta dashed lines
✰ The four blue dashed lines
(iii) The required lines are shown in fig.4.182(b)
♦ Altogether, there are six lines
♦ The six hybrid orbitals will be oriented along these lines
♦ This is shown in fig.c
■ Thus we get the 'sp3d2 hybridized S atom'
3. Let us write three 'important points to remember' about fig.4.182:
(i) The 'plane of the base square' is exactly at the middle of the structure
♦ So this plane is called 'equatorial plane'
(ii) All the four green dashed lines lie on the 'plane of the base square'
♦ So the four orbitals along the green lines are called 'equatorial orbitals'
♦ These orbitals are at an angular distance of 90o apart
(iii) The two red dashed lines form the axis of the structure
♦ So the two orbitals along the red lines are called 'axial orbitals'
♦ They are at an angular distance of 180o apart
4. So we can write:
♦ The 'sp3d2 hybridized S atom' consists of two items:
✰ The six hybrid orbitals
✰ The nucleus (small red sphere)
• In the fig.4.178(c), the 'smaller lobes of hybrid orbitals' are not shown
♦ This is to reduce congestion and thus obtain greater clarity
5. Distribution of electrons in the hybrid orbitals:
• This can be written in 3 steps:
(i) We know that, the sp3d2 hybrid orbitals are formed from 'one s orbital', 'three p orbitals' and 'two d orbitals'
(ii) In our present case of S atom, they are: 'one 3s orbital', 'three 3p orbitals' and 'two 3d orbitals'
(iii) Before the hybridization, the orbitals mentioned in (ii) carry a total of six electrons
♦ After hybridization, the orbitals mentioned in (ii) will no longer exist
♦ Then what will happen to the six electrons?
Answer:
• The six electrons will be distributed among the six sp3d2 hybrid orbitals
♦ So each hybrid orbital will carry one electron
♦ This is indicated by the arrows in fig.4.182(c) above
6. So in fig.4.178(c), we have:
♦ Six half filled hybrid orbitals• Now bonding with Cl atoms can begin
• First we will write some basic details about the Cl atom. It can be written in 2 steps:
(We have already seen the two steps in the previous section and in the previous case of PCl5. But we will write them again)
(i) The electronic configuration of Cl is: [Ne]3s23p5
• Expanding the valence orbitals, the configuration becomes: [Ne]3s2 3px2 3py2 3pz1
(ii) It is clear that, the 3pz of Cl is half filled
• This 3pz needs one more electron
• We saw it in fig.4.166 in the previous section. It is shown again below:
 |
| Fig.4.166 |
♦ It has two arrows
• The py orbital is shown in green color
♦ It has two arrows
• The pz orbital is shown in blue color
♦ It has only one arrow
• The 3s orbital is represented by the cyan sphere
♦ It has two arrows
7. One Cl atom will come and overlap with each of the six half filled orbitals of the S atom
• So a total of six Cl atoms will be coming
♦ In each of those Cl atoms, it is the pz which enters into bonding
♦ This is shown in fig.4.183(a) below:
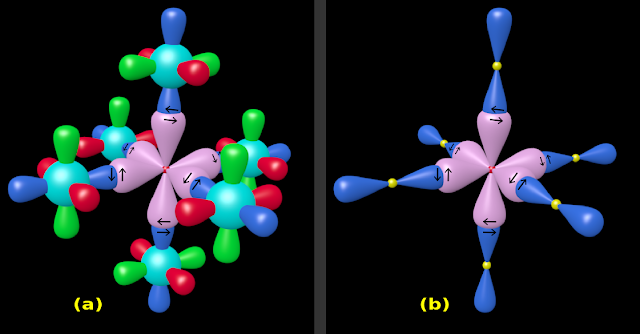 |
| Fig.4.183 |
8. The electron clouds of Cl:
(i) The red, green, blue and cyan regions around the Cl nucleus are mere electron clouds
♦ To define the shape of a molecule:
✰ We do not consider the positions of electrons or electron clouds
✰ We consider only the positions of 'nuclei of atoms' in that molecule
(ii) So we do not need to show the orbitals (electron clouds) of the Cl atoms. We can delete them
• However, we will retain the pz orbital. This will convey the fact that, it is 'a p orbital of Cl' that bonds with the S atom
(iii) The final structure is shown in fig.4.183(b) above
• The small yellow spheres represent the nuclei of Cl atoms
• Let us write the salient features of this model. It can be written in 3 steps:
(i) There is a total of six S-F bonds
♦ Four of them lie on the equatorial plane
✰ Along the diagonals of the base square
♦ The remaining two lie along the 'axis of the octahedron'
(ii) So there are:
♦ Four equatorial bonds
♦ Two axial bonds
(iii) The angle between
♦ Any two equatorial bonds is 90o
♦ The two axial bonds is 180o
♦ Any equatorial bond and axial bond is 90o
10. The 2D representation is shown below:
• Recall that, we saw the same 2D representation when we analysed SF6 using VSEPR theory. See fig.4.98(c) in section 4.16
• There we saw the reason for giving the solid triangle, dashed triangle etc.,
11. Note that, all the bonds in SF6 are sigma bonds
• We have seen the a number of examples in which the central atom undergoes some sort of hybridization
• Let us now write the salient features of hybridization
• We have already seen the application of those features in the various examples
• However, it is better to prepare a compilation of the features
• There are four salient features
1. The 'number of hybrid orbitals' is equal to the 'number of atomic orbitals that get hybridized'
Application example:
• In sp3d hybridization, five atomic orbitals are involved:
♦ One s orbital
♦ Three p orbitals
♦ One d orbital
• So the total number of participating orbitals is five
• After the hybridization, we get five hybrid orbitals
2. The hybrid orbitals are always equivalent in energy and shape
Application example:
• Regarding shape:
♦ In all the examples, we see the same hybrid orbitals
✰ Each of those orbitals has a larger lobe and a smaller lobe
• Regarding energy:
♦ In CH4, four hybrid orbitals are produced
✰ All four have the same energy
♦ In PCl5, five hybrid orbitals are produced
✰ All five have the same energy
3. The hybrid orbitals are more effective in forming stable bonds than pure atomic orbitals
Application example:
• Nitrogen has three half filled pure orbitals
♦ So it can form NH3
♦ But it prefers to use hybrid orbitals
♦ See section 4.28
4. The hybrid orbitals push each other as far away as possible
♦ This is to reduce repulsion
♦ So they orient in some specific directions
♦ Since there are specific directions, we are able to find the shapes of various molecules
Application example:
• As a result of hybridization in CH4, four hybrid orbitals are produced
♦ Each of those hybrid orbitals try to push the other as far away as possible
♦ This ‘pushing’ results in a tetrahedral shape
♦ Thus the shape of CH4 is tetrahedral
Now we will write the four important conditions for hybridization
Application:
• We need not consider the inner orbitals while analyzing hybridization in an atom
• We need to consider only the valence shell orbitals
2. The orbitals undergoing hybridization should have almost equal energy
Application example:
• In PCl5, we saw that 4s, 3p and 3d of P cannot hybridize together
♦ This is because, their energies differ vastly
♦ See fig.4.176 of the previous section
3. Promotion of electron is not an essential condition prior to hybridization
Application example:
• In PCl5, we saw that, one electron in the 3s orbital gets promoted to a 3d orbital
♦ This promotion may take place even after the formation of the five hybrid orbitals
4. It is not necessary that, only half filled orbitals participate in hybridization. In some cases, even filled orbitals in the valence shell take part in hybridization
Application example:
• In NH3, the 2s orbital is completely filled
♦ Even then it participates in hybridization
♦ See fig.4.160 in section 4.28
Now we can write the definition of hybridization. It can be written in 2 steps
1. Hybridization is the process of intermixing of orbitals
♦ Those participating orbitals should have only ‘slightly different’ energies
♦ During the hybridization, ‘redistribution of energies’ take place
2. After hybridization, a set of new orbitals is produced
♦ Each of those new orbitals will have the same energy
♦ Each of those new orbitals will have the same shape
1. Hybridization is the process of intermixing of orbitals
♦ Those participating orbitals should have only ‘slightly different’ energies
♦ During the hybridization, ‘redistribution of energies’ take place
2. After hybridization, a set of new orbitals is produced
♦ Each of those new orbitals will have the same energy
♦ Each of those new orbitals will have the same shape
• We have completed a discussion on valence bond theory and hybridization concept
• We will see the solved examples (section 4.40) after completing a discussion on molecular orbital theory also
• In the next few sections, we will be discussing the molecular orbital theory
• We will see the solved examples (section 4.40) after completing a discussion on molecular orbital theory also
• In the next few sections, we will be discussing the molecular orbital theory


No comments:
Post a Comment