• In the previous section 4.27, we saw the structure of C2H4
♦ We also discussed pi bonds in triple bonds
• The compounds that we saw in the previous three sections were all organic compounds: CH4, C2H6, C2H4 and C2H2
• Those organic compounds helped us to get a good understanding about sp3, sp2 and sp hybridization
♦ They helped us to learned about sigma and pi bonds also
• The concept of hybridization can be used to explain the structure of inorganic molecules also
♦ In this section, we will see the structure of NH3 (ammonia) and H2O (water)
Structure of NH3
• Fig.4.160(a) below shows the Lewis dot structure of NH3
• The details about the model of NH3 can be written in 9 steps:
1. The N atom in NH3 is sp3 hybridized
• Let us see how this sp3 hybridization is achieved. It can be written in 2 steps:
(i) Fig.4.160(b) below shows the orbitals in the valence shell of N
• We see:
♦ One 2s orbital
✰ It is completely filled
♦ Three 2p orbitals
✰ They are half filled
(ii) The total four orbitals mix together to form four 'sp3 hybrid orbitals'
2. Now a question arises in our minds. The question and it's answer can be detailed in 4 steps:
(i) The N atom already has three half filled orbitals
♦ They are: 2px, 2py and 2pz
(ii) The three H atoms can bond with those half filled orbitals
(iii) Then why would hybridization take place?
(iv) The answer can be written in five steps (a to e):
(a) 2px, 2py and 2pz are atomic orbitals
(b) But the four sp3 orbitals are hybrid orbitals
(c) Bonds with hybrid orbitals are more stable and stronger
✰ This is because, the hybrid orbitals have greater electron density towards one tip
✰ This is the tip of the larger lobe
✰ We see that, the lobe becomes larger towards the tip
✰ The bond is formed at this tip
✰ A 'bond at a region of greater electron density', will naturally be stronger
(d) In an 'ordinary atomic orbital', the electron density is uniform
✰ This is evident from the 'equal size' of the two lobes
(e) So the N atom will prefer to use hybrid orbitals rather than atomic orbitals
(We will see a more detailed mathematical explanation in higher classes)
3. We know that:
♦ In sp3 hybridization, there will be four hybrid orbitals
✰ Together, they form a tetrahedral shape
• This is shown in fig.4.161(a) below:
• Let us write three 'important points to remember' about fig.a:
(i) The four sp3 hybrid orbitals are at an angular distance of 109.5o apart (This is the characteristic angle of any tetrahedron)
(ii) The nucleus of the N atom is shown as a small red sphere
(iii) We know that, each hybrid orbital have a larger lobe and a smaller lobe
♦ Here we deliberately choose not to show the smaller lobes. This is for better clarity
4. So we can write:
• The N atom now consists of two items:
(i) Four sp3 hybridized orbitals
(ii) Nucleus (the small red sphere)
5. Distribution of electrons in the hybrid orbitals:
• This can be written in 3 steps:
(i) We know that, the sp3 hybrid orbitals are formed from 'one s orbital' and 'three p orbitals'
(ii) In our present case of N atom, they are: 'one 2s orbital' and 'three 2p orbitals'
(iii) Before the hybridization, the orbitals mentioned in (ii) carry a total of five electrons
♦ After hybridization, the orbitals mentioned in (ii) will no longer exist
♦ Then what will happen to the five electrons?
Answer:
• The five electrons will be distributed among the four sp3 hybrid orbitals
♦ So 'one out of the four hybrid orbitals' will carry two electrons
✰ This is the upward pointing orbital in fig.4.161(a) above
✰ There are two arrows in this orbital
♦ Each of the 'three remaining orbitals' will carry one electron each
✰ In fig.4.161(a), there is only one arrow in each of these orbitals
6. So in fig.4.161(a), we have:
♦ One hybrid orbital which is completely filled
♦ Three hybrid orbitals which are half filled
• Now bonding with H atoms can begin:
♦ One H atom will come and overlap with each of the three half filled orbitals of the N atom
♦ This is shown in fig.4.161(b)
7. Thus we get the model of the NH3 molecule
• Let us write the salient features of this model. It can be written in 7 steps:
(i) The three H atoms are situated at the three corners of a triangle
♦ This triangle is the base of a triangular pyramid
(ii) The apex of the pyramid is occupied by the 'tip of the completely filled hybrid orbital'
♦ In other words, the apex is occupied by a lone pair
(iii) The N atom is situated at the 'center of gravity' of the pyramid
(iv) Now, to define the shape of a molecule, we can consider only the positions of the atoms
♦ We cannot consider the positions of lone pairs
(v) Thus we consider the positions of the three 'H atoms' and the 'N atom'
♦ Together, those four atoms form a 'pyramidal shape'
(vi) The angle between any two bonds should be 109.5o
♦ Because, the 'tetrahedral shape' is used as the 'basic shape' to derive the 'final pyramidal shape'
(vii) But due to the pushing effect of the lone pair, the angle reduces from 109.5o to 107o
♦ We saw this 'pushing by lone pair' earlier when we discussed VSEPR theory
♦ The 'pushing' is indicated by the yellow arrows in fig.4.106(a) in section 4.18
♦ Steps explaining the derivation of the 'final pyramidal shape' from the 'basic tetrahedral shape' are also given in that section
8. We have already seen the 2D representation of the NH3 molecule when we discussed the VSEPR theory. See fig.4.107(b) in section 4.18
9. Note that, all the bonds in NH3 are sigma bonds
Structure of H2O
• Fig.4.162(a) below shows the Lewis dot structure of H2O
• The details about the model of H2O can be written in 9 steps:
1. The H atom in H2O is sp3 hybridized
• Let us see how this sp3 hybridization is achieved. It can be written in 2 steps:
(i) Fig.4.160(b) below shows the orbitals in the valence shell of O
• We see:
♦ One 2s orbital
✰ It is completely filled
♦ Three 2p orbitals
✰ One of them is completely filled
✰ The remaining two are half filled
(ii) The total four orbitals mix together to form four 'sp3 hybrid orbitals'
2. Now a question arises in our minds. The question and it's answer can be detailed in 4 steps:
(i) The O atom already has two half filled orbitals
♦ They are: 2py and 2pz
(ii) The two H atoms can bond with those two half filled orbitals
(iii) Then why would hybridization take place?
(iv) The answer can be written in five steps (a to e):
(a) 2py and 2pz are atomic orbitals
(b) But the four sp3 orbitals are hybrid orbitals
(c) Bonds with hybrid orbitals are more stable and stronger
✰ This is because, the hybrid orbitals have greater electron density towards one tip
✰ This is the tip of the larger lobe
✰ We see that, the lobe becomes larger towards the tip
✰ The bond is formed at this tip
✰ A 'bond at a region of greater electron density', will naturally be stronger
(d) In an 'ordinary atomic orbital', the electron density is uniform
✰ This is evident from the 'equal size' of the two lobes
(e) So the O atom will prefer to use hybrid orbitals rather than atomic orbitals
(We will see a more detailed mathematical explanation in higher classes)
3. We know that:
♦ In sp3 hybridization, there will be four hybrid orbitals
✰ Together, they form a tetrahedral shape
• This is shown in fig.4.163(a) below:
• Let us write three 'important points to remember' about fig.a:
(i) The four sp3 hybrid orbitals are at an angular distance of 109.5o apart (This is the characteristic angle of any tetrahedron)
(ii) The nucleus of the O atom is shown as a small red sphere
(iii) We know that, each hybrid orbital have a larger lobe and a smaller lobe
♦ Here we deliberately choose not to show the smaller lobes. This is for better clarity
4. So we can write:
• The O atom now consists of two items:
(i) Four sp3 hybridized orbitals
(ii) Nucleus (the small red sphere)
5. Distribution of electrons in the hybrid orbitals:
• This can be written in 3 steps:
(i) We know that, the sp3 hybrid orbitals are formed from 'one s orbital' and 'three p orbitals'
(ii) In our present case of O atom, they are: 'one 2s orbital' and 'three 2p orbitals'
(iii) Before the hybridization, the orbitals mentioned in (ii) carry a total of six electrons
♦ After hybridization, the orbitals mentioned in (ii) will no longer exist
♦ Then what will happen to the six electrons?
Answer:
• The six electrons will be distributed among the four sp3 hybrid orbitals
♦ So 'two out of the four hybrid orbitals' will carry two electrons each
✰ They are the 'upward pointing orbital' and the 'rear orbital' in fig.4.163(a) above
✰ There are two arrows in each of those orbitals
♦ Each of the 'two remaining orbitals' will carry one electron each
✰ In fig.4.163(a), there is only one arrow in each of those orbitals
6. So in fig.4.163(a), we have:
♦ Two hybrid orbitals which are completely filled
♦ Two hybrid orbitals which are half filled
• Now bonding with H atoms can begin:
♦ One H atom will come and overlap with each of the two half filled orbitals of the O atom
♦ This is shown in fig.4.163(b)
7. Thus we get the model of the H2O molecule
• Let us write the salient features of this model. It can be written in 7 steps:
(i) The two H atoms are situated at the two corners of a triangle
♦ This triangle is the base of a triangular pyramid
• The third corner of the base triangle is occupied by the 'tip of one of the completely filled hybrid orbitals'
♦ In other words, the third corner is occupied by a lone pair
(iii) The O atom is situated at the 'center of gravity' of the pyramid
(iv) Now, to define the shape of a molecule, we can consider only the positions of the atoms
♦ We cannot consider the positions of lone pairs
(v) Thus we consider the positions of the two 'H atoms' and the 'O atom'
♦ Together, those three atoms form a 'V shape'
(vi) The angle between any two bonds should be 109.5o
♦ Because, the 'tetrahedral shape' is used as the 'basic shape' to derive the 'final V shape'
(vii) But due to the pushing effect of the lone pairs, the angle reduces from 109.5o to 105o
♦ We saw this 'pushing by lone pair' earlier when we discussed VSEPR theory
♦ The 'pushing' is indicated by the cyan arrow in fig.4.103(a) in section 4.17
♦ Steps explaining the derivation of the 'final V shape' from the 'basic tetrahedral shape' are also given in that section
8. We have already seen the 2D representation of the H2O molecule when we discussed the VSEPR theory. See fig.4.104 in section 4.17
9. Note that, all the bonds in H2O are sigma bonds
♦ We also discussed pi bonds in triple bonds
• The compounds that we saw in the previous three sections were all organic compounds: CH4, C2H6, C2H4 and C2H2
• Those organic compounds helped us to get a good understanding about sp3, sp2 and sp hybridization
♦ They helped us to learned about sigma and pi bonds also
• The concept of hybridization can be used to explain the structure of inorganic molecules also
♦ In this section, we will see the structure of NH3 (ammonia) and H2O (water)
Structure of NH3
• Fig.4.160(a) below shows the Lewis dot structure of NH3
• The details about the model of NH3 can be written in 9 steps:
1. The N atom in NH3 is sp3 hybridized
• Let us see how this sp3 hybridization is achieved. It can be written in 2 steps:
(i) Fig.4.160(b) below shows the orbitals in the valence shell of N
 |
| Fig.4.160 |
♦ One 2s orbital
✰ It is completely filled
♦ Three 2p orbitals
✰ They are half filled
(ii) The total four orbitals mix together to form four 'sp3 hybrid orbitals'
2. Now a question arises in our minds. The question and it's answer can be detailed in 4 steps:
(i) The N atom already has three half filled orbitals
♦ They are: 2px, 2py and 2pz
(ii) The three H atoms can bond with those half filled orbitals
(iii) Then why would hybridization take place?
(iv) The answer can be written in five steps (a to e):
(a) 2px, 2py and 2pz are atomic orbitals
(b) But the four sp3 orbitals are hybrid orbitals
(c) Bonds with hybrid orbitals are more stable and stronger
✰ This is because, the hybrid orbitals have greater electron density towards one tip
✰ This is the tip of the larger lobe
✰ We see that, the lobe becomes larger towards the tip
✰ The bond is formed at this tip
✰ A 'bond at a region of greater electron density', will naturally be stronger
(d) In an 'ordinary atomic orbital', the electron density is uniform
✰ This is evident from the 'equal size' of the two lobes
(e) So the N atom will prefer to use hybrid orbitals rather than atomic orbitals
(We will see a more detailed mathematical explanation in higher classes)
3. We know that:
♦ In sp3 hybridization, there will be four hybrid orbitals
✰ Together, they form a tetrahedral shape
• This is shown in fig.4.161(a) below:
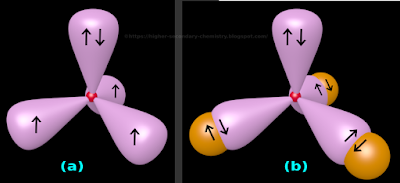 |
| Fig.4.161 |
(i) The four sp3 hybrid orbitals are at an angular distance of 109.5o apart (This is the characteristic angle of any tetrahedron)
(ii) The nucleus of the N atom is shown as a small red sphere
(iii) We know that, each hybrid orbital have a larger lobe and a smaller lobe
♦ Here we deliberately choose not to show the smaller lobes. This is for better clarity
4. So we can write:
• The N atom now consists of two items:
(i) Four sp3 hybridized orbitals
(ii) Nucleus (the small red sphere)
5. Distribution of electrons in the hybrid orbitals:
• This can be written in 3 steps:
(i) We know that, the sp3 hybrid orbitals are formed from 'one s orbital' and 'three p orbitals'
(ii) In our present case of N atom, they are: 'one 2s orbital' and 'three 2p orbitals'
(iii) Before the hybridization, the orbitals mentioned in (ii) carry a total of five electrons
♦ After hybridization, the orbitals mentioned in (ii) will no longer exist
♦ Then what will happen to the five electrons?
Answer:
• The five electrons will be distributed among the four sp3 hybrid orbitals
♦ So 'one out of the four hybrid orbitals' will carry two electrons
✰ This is the upward pointing orbital in fig.4.161(a) above
✰ There are two arrows in this orbital
♦ Each of the 'three remaining orbitals' will carry one electron each
✰ In fig.4.161(a), there is only one arrow in each of these orbitals
6. So in fig.4.161(a), we have:
♦ One hybrid orbital which is completely filled
♦ Three hybrid orbitals which are half filled
• Now bonding with H atoms can begin:
♦ One H atom will come and overlap with each of the three half filled orbitals of the N atom
♦ This is shown in fig.4.161(b)
7. Thus we get the model of the NH3 molecule
• Let us write the salient features of this model. It can be written in 7 steps:
(i) The three H atoms are situated at the three corners of a triangle
♦ This triangle is the base of a triangular pyramid
(ii) The apex of the pyramid is occupied by the 'tip of the completely filled hybrid orbital'
♦ In other words, the apex is occupied by a lone pair
(iii) The N atom is situated at the 'center of gravity' of the pyramid
(iv) Now, to define the shape of a molecule, we can consider only the positions of the atoms
♦ We cannot consider the positions of lone pairs
(v) Thus we consider the positions of the three 'H atoms' and the 'N atom'
♦ Together, those four atoms form a 'pyramidal shape'
(vi) The angle between any two bonds should be 109.5o
♦ Because, the 'tetrahedral shape' is used as the 'basic shape' to derive the 'final pyramidal shape'
(vii) But due to the pushing effect of the lone pair, the angle reduces from 109.5o to 107o
♦ We saw this 'pushing by lone pair' earlier when we discussed VSEPR theory
♦ The 'pushing' is indicated by the yellow arrows in fig.4.106(a) in section 4.18
♦ Steps explaining the derivation of the 'final pyramidal shape' from the 'basic tetrahedral shape' are also given in that section
8. We have already seen the 2D representation of the NH3 molecule when we discussed the VSEPR theory. See fig.4.107(b) in section 4.18
9. Note that, all the bonds in NH3 are sigma bonds
Structure of H2O
• Fig.4.162(a) below shows the Lewis dot structure of H2O
• The details about the model of H2O can be written in 9 steps:
1. The H atom in H2O is sp3 hybridized
• Let us see how this sp3 hybridization is achieved. It can be written in 2 steps:
(i) Fig.4.160(b) below shows the orbitals in the valence shell of O
 |
| Fig.4.162 |
♦ One 2s orbital
✰ It is completely filled
♦ Three 2p orbitals
✰ One of them is completely filled
✰ The remaining two are half filled
(ii) The total four orbitals mix together to form four 'sp3 hybrid orbitals'
2. Now a question arises in our minds. The question and it's answer can be detailed in 4 steps:
(i) The O atom already has two half filled orbitals
♦ They are: 2py and 2pz
(ii) The two H atoms can bond with those two half filled orbitals
(iii) Then why would hybridization take place?
(iv) The answer can be written in five steps (a to e):
(a) 2py and 2pz are atomic orbitals
(b) But the four sp3 orbitals are hybrid orbitals
(c) Bonds with hybrid orbitals are more stable and stronger
✰ This is because, the hybrid orbitals have greater electron density towards one tip
✰ This is the tip of the larger lobe
✰ We see that, the lobe becomes larger towards the tip
✰ The bond is formed at this tip
✰ A 'bond at a region of greater electron density', will naturally be stronger
(d) In an 'ordinary atomic orbital', the electron density is uniform
✰ This is evident from the 'equal size' of the two lobes
(e) So the O atom will prefer to use hybrid orbitals rather than atomic orbitals
(We will see a more detailed mathematical explanation in higher classes)
3. We know that:
♦ In sp3 hybridization, there will be four hybrid orbitals
✰ Together, they form a tetrahedral shape
• This is shown in fig.4.163(a) below:
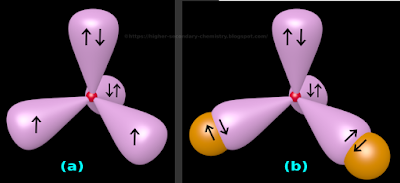 |
| Fig.4.163 |
(i) The four sp3 hybrid orbitals are at an angular distance of 109.5o apart (This is the characteristic angle of any tetrahedron)
(ii) The nucleus of the O atom is shown as a small red sphere
(iii) We know that, each hybrid orbital have a larger lobe and a smaller lobe
♦ Here we deliberately choose not to show the smaller lobes. This is for better clarity
4. So we can write:
• The O atom now consists of two items:
(i) Four sp3 hybridized orbitals
(ii) Nucleus (the small red sphere)
5. Distribution of electrons in the hybrid orbitals:
• This can be written in 3 steps:
(i) We know that, the sp3 hybrid orbitals are formed from 'one s orbital' and 'three p orbitals'
(ii) In our present case of O atom, they are: 'one 2s orbital' and 'three 2p orbitals'
(iii) Before the hybridization, the orbitals mentioned in (ii) carry a total of six electrons
♦ After hybridization, the orbitals mentioned in (ii) will no longer exist
♦ Then what will happen to the six electrons?
Answer:
• The six electrons will be distributed among the four sp3 hybrid orbitals
♦ So 'two out of the four hybrid orbitals' will carry two electrons each
✰ They are the 'upward pointing orbital' and the 'rear orbital' in fig.4.163(a) above
✰ There are two arrows in each of those orbitals
♦ Each of the 'two remaining orbitals' will carry one electron each
✰ In fig.4.163(a), there is only one arrow in each of those orbitals
6. So in fig.4.163(a), we have:
♦ Two hybrid orbitals which are completely filled
♦ Two hybrid orbitals which are half filled
• Now bonding with H atoms can begin:
♦ One H atom will come and overlap with each of the two half filled orbitals of the O atom
♦ This is shown in fig.4.163(b)
7. Thus we get the model of the H2O molecule
• Let us write the salient features of this model. It can be written in 7 steps:
(i) The two H atoms are situated at the two corners of a triangle
♦ This triangle is the base of a triangular pyramid
• The third corner of the base triangle is occupied by the 'tip of one of the completely filled hybrid orbitals'
♦ In other words, the third corner is occupied by a lone pair
(ii) The apex of the pyramid is occupied by the 'tip of the completely filled hybrid orbital'
♦ In other words, the apex is occupied by a lone pair (iii) The O atom is situated at the 'center of gravity' of the pyramid
(iv) Now, to define the shape of a molecule, we can consider only the positions of the atoms
♦ We cannot consider the positions of lone pairs
(v) Thus we consider the positions of the two 'H atoms' and the 'O atom'
♦ Together, those three atoms form a 'V shape'
(vi) The angle between any two bonds should be 109.5o
♦ Because, the 'tetrahedral shape' is used as the 'basic shape' to derive the 'final V shape'
(vii) But due to the pushing effect of the lone pairs, the angle reduces from 109.5o to 105o
♦ We saw this 'pushing by lone pair' earlier when we discussed VSEPR theory
♦ The 'pushing' is indicated by the cyan arrow in fig.4.103(a) in section 4.17
♦ Steps explaining the derivation of the 'final V shape' from the 'basic tetrahedral shape' are also given in that section
8. We have already seen the 2D representation of the H2O molecule when we discussed the VSEPR theory. See fig.4.104 in section 4.17
9. Note that, all the bonds in H2O are sigma bonds
• In the next section, we will see the structures of BCl3 and BeCl2
No comments:
Post a Comment