• In the previous section 4.29, we saw the structure of BCl3 and BeCl2. In this section, we will see the hybridization involving d orbitals
• In the cases that we saw so far, the hybridization of the central atom involved s and p orbitals only
• When we consider the elements in the third period, we will have to deal with d orbitals also
♦ That means, the d orbitals will also mix together with s and p orbitals
■ For example:
• In sp3d hybridization, there will be one s, three p and one d orbitals
♦ That is., (s + p + p + p + d)
• Let us see the various possibilities when d orbitals are also involved in the hybridization. It can be written in 3 steps:
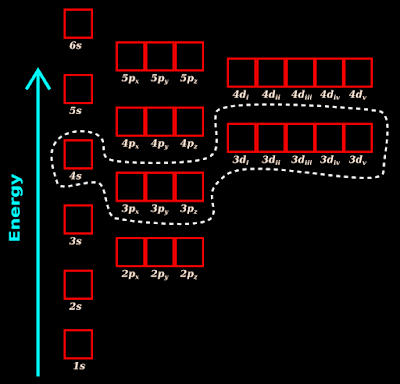
1. Fig.4.173 below shows the energy levels of various orbitals
(Note: The actual names of the five d-orbitals are: $\mathbf\small{d_{xy},\;d_{xz},\;d_{yz},\;d_{x^2-y^2},\;\rm{and}\;d_{z^2}}$. In the figs. below, they are named as di, dii, diii, div and dv. This is for saving space)
2. Let us compare some energies
• The lowest d-orbitals are the 3d orbitals. So we will investigate the comparisons related to those 3d orbitals:
• We see that:
(i) The energy of 3d is comparable to the energy of 3s and 3p
(ii) The energy of 3d is again comparable to the energy of 4s and 4p
(iii) The energy of 3d is not comparable to the energy of 4s and 3p
♦ This is because, there is much difference between the energies of those orbitals
✰ The 4s sub-shell is in a 'higher main-shell'
✰ The 3p and 3d sub-shells are in a 'lower main-shell'
3. Based on the above comparison, we can write the 'combinations which are possible' and 'combinations which are not possible'
(i) The following two combinations are possible:
♦ Hybridization involving 3s, 3p and 3d orbitals [based on 2(i)]
✰ This is shown in fig.4.174 below
♦ Hybridization involving 3d, 4s and 4p orbitals [based on 2(ii)]
✰ This is shown in fig.4.175 further below
(ii) The following combination is not possible:
♦ Hybridization involving 3p, 3d and 4s [based on 2(iii)]
✰ This is shown in fig.4.176 further below
✰ The outline is drawn using dashed lines to indicate that, the combination is not possible
Structure of PCl5
• When we discussed VSEPR theory, we saw the Lewis dot structure of PCl5
♦ See fig.4.93(c) in section 4.15
♦ All the five P-Cl bonds are single bonds
• For convenience, that fig. is shown again below:
• The details about the shape of PCl5 can be written in 12 steps:
1. The P atom in PCl5 is sp3d hybridized
• Let us see how this sp3d hybridization is achieved. It can be written in 2 steps:
(i) Fig.4.177(a) below shows the orbitals of P
(ii) When enough energy is given, one electron in the 3s orbital jumps to the 3di orbital
• Thus we get five half filled orbitals. This is shown in fig.b
(iii) The 3s orbital mixes together with 3px, 3py, 3pz and 3di. This is also shown in fig.b
(iv) Since there is one s-orbital, three p-orbitals, and one d orbital, it is a sp3d hybridization
2. We know that:
♦ In sp3d hybridization, there will be five resulting hybrid orbitals
• Together, they form a triangular bipyramidal shape
♦ This can be explained in 3 steps:
(i) Fig.4.178(a) below, shows the outlines of a triangular bipyramid
♦ The base triangle is shown in blue dashed lines
✰ The small red sphere is the nucleus of the P atom
✰ This nucleus is situated a the 'center of gravity' of the base triangle
♦ Three green dashed lines radiate out from the nucleus
✰ These lines are at an angular distance of 120o apart
♦ Two red dashed lines radiate upwards and downwards from the nucleus
✰ These lines together form the axis of the bipyramid
♦ The magenta dashed lines are the sloping edges of the bipyramid
(ii) Lines which are required:
♦ We require the following lines:
✰ The three green dashed lines
✰ The two red dashed lines (these two, together form the axis)
♦ We do not require the following lines:
✰ The six magenta dashed lines
✰ The three blue dashed lines
(iii) The required lines are shown in fig.4.178(b)
♦ Altogether, there are five lines
♦ The five hybrid orbitals will be oriented along these lines
♦ This is shown in fig.c
■ Thus we get the 'sp3d hybridized P atom'
3. Let us write three 'important points to remember' about fig.4.178:
(i) The 'plane of the base triangle' is exactly at the middle of the structure
♦ So this plane is called 'equatorial plane'
(ii) All the three green dashed lines lie on the 'plane of the base triangle'
♦ So the three orbitals along the green lines are called 'equatorial orbitals'
♦ These orbitals are at an angular distance of 120o apart
(iii) The two red dashed lines form the axis of the structure
♦ So the two orbitals along the red lines are called 'axial orbitals'
♦ They are at an angular distance of 180o apart
4. So we can write:
♦ The 'sp3d hybridized P atom' consists of two items:
✰ The five hybrid orbitals
✰ The nucleus (small red sphere)
• In the fig.4.178(c), the 'smaller lobes of hybrid orbitals' are not shown
♦ This is to reduce congestion and thus obtain greater clarity
5. Distribution of electrons in the hybrid orbitals:
• This can be written in 3 steps:
(i) We know that, the sp3d hybrid orbitals are formed from 'one s orbital', 'three p orbitals' and 'one d orbital'
(ii) In our present case of P atom, they are: 'one 3s orbital', 'three 3p orbitals' and 'one 3d orbital'
(iii) Before the hybridization, the orbitals mentioned in (ii) carry a total of five electrons
♦ After hybridization, the orbitals mentioned in (ii) will no longer exist
♦ Then what will happen to the three electrons?
Answer:
• The five electrons will be distributed among the five sp3d hybrid orbitals
♦ So each hybrid orbital will carry one electron
♦ This is indicated by the arrows in fig.4.178(c) above
• Now bonding with Cl atoms can begin
• First we will write some basic details about the Cl atom. It can be written in 2 steps:
(We have already seen the two steps in the previous section. But we will write them again)
(i) The electronic configuration of Cl is: [Ne]3s23p5
• Expanding the valence orbitals, the configuration becomes: [Ne]3s2 3px2 3py2 3pz1
(ii) It is clear that, the 3pz of Cl is half filled
• This 3pz needs one more electron
• We saw it in fig.4.166 in the previous section. It is shown again below:
• The px orbital is shown in red color
♦ It has two arrows
• The py orbital is shown in green color
♦ It has two arrows
• The pz orbital is shown in blue color
♦ It has only one arrow
• The 3s orbital is represented by the cyan sphere
♦ It has two arrows
7. One Cl atom will come and overlap with each of the five half filled orbitals of the P atom
• So a total of five Cl atoms will be coming
♦ In each of those Cl atoms, it is the pz which enters into bonding
♦ This is shown in fig.4.179(a) below:
• In the fig.4.179(a) above, the 'pz orbitals of Cl' have become completely filled
8. The electron clouds of Cl:
(i) The red, green, blue and cyan regions around the Cl nucleus are mere electron clouds
♦ To define the shape of a molecule:
✰ We do not consider the positions of electrons or electron clouds
✰ We consider only the positions of 'nuclei of atoms' in that molecule
(ii) So we do not need to show the orbitals (electron clouds) of the Cl atoms. We can delete them
• However, we will retain the pz orbital. This will convey the fact that, it is 'a p orbital of Cl' that bonds with the P atom
(iii) The final structure is shown in fig.4.179(b) above
• The small yellow spheres represent the nuclei of Cl atoms
9. Thus we get the model of the PCl5 molecule
• Let us write the salient features of this model. It can be written in 3 steps:
(i) There is a total of five P-Cl bonds
♦ Three of them lie on the equatorial plane
♦ The remaining two lie along the 'axis of the bipyramid'
(ii) So there are:
♦ Three equatorial bonds
♦ Two axial bonds
(iii) The angle between
♦ Any two equatorial bonds is 120o
♦ The two axial bonds is 180o
♦ Any equatorial bond and axial bond is 90o
10. Now we will see an interesting feature about bond lengths
(i) Consider fig.4.178(b) that we saw earlier
♦ There is a total of 5 dashed lines
♦ Those five lines give us the directions of the bonds
(ii) When the PCl5 molecule is formed, each of those five lines will contain a bond pair of electrons
♦ Repulsion will occur between those electron pairs
♦ We can mark those repulsions in the fig.4.178(b)
♦ Fig.4.180(a) below, is one such fig.
✰ It shows the repulsion in the equatorial plane (horizontal plane)
♦ Fig.4.180(b) is another such fig.
✰ It shows the repulsion in the vertical planes
(iii) We can ignore fig.a
• Because, the red double headed arrows will cancel each other out
(iv) But we cannot ignore fig.b
• Let us write the reason. It can be written in three steps (a), (b) and (c):
(a) There are three blue arrows above the equatorial plane
✰ Those arrows will push the 'top most electron pair' away from the equatorial plane
✰ So that electron pair will move further upwards
✰ As a result, the 'bond length' of the 'upper axial bond' will increase
(b) There are three blue arrows below the equatorial plane
✰ Those arrows will push the 'bottom most electron pair' away from the equatorial plane
✰ So that electron pair will move further downwards
✰ As a result, the 'bond length' of the 'lower axial bond' will increase
(c) The difference in bond lengths is shown in fig.4.180(c) above
11. Fig.4.180(c) shows the 2D representation also
• Recall that, we saw the same 2D representation when we analysed PCl5 using VSEPR theory. See fig.4.93(c) in section 4.15
• There we saw the reason for giving the solid triangle, dashed triangle etc.,
12. Note that, all the bonds in PCl5 are sigma bonds
♦ PF5 also has the same trigonal bipyramidal structure
♦ That means, PF5 will have the same structure as shown in fig.4.179(b) above
2. Note that Cl and F belongs to the same group in the periodic table
♦ The electronic configuration of Cl is [Ne]3s2 3px2 3py2 3pz1
♦ The electronic configuration of F is [He]2s2 2px2 2py2 2pz1
3. Now the reader can write the necessary steps to arrive at the structure of PF5
[Hint: In the case of PF5, the blue orbitals in fig.4.179(b) will be 2pz of F atoms]
• In the cases that we saw so far, the hybridization of the central atom involved s and p orbitals only
• When we consider the elements in the third period, we will have to deal with d orbitals also
♦ That means, the d orbitals will also mix together with s and p orbitals
■ For example:
• In sp3d hybridization, there will be one s, three p and one d orbitals
♦ That is., (s + p + p + p + d)
• Let us see the various possibilities when d orbitals are also involved in the hybridization. It can be written in 3 steps:
1. Fig.4.173 below shows the energy levels of various orbitals
(Note: The actual names of the five d-orbitals are: $\mathbf\small{d_{xy},\;d_{xz},\;d_{yz},\;d_{x^2-y^2},\;\rm{and}\;d_{z^2}}$. In the figs. below, they are named as di, dii, diii, div and dv. This is for saving space)
 |
| Fig.4.173 |
• The lowest d-orbitals are the 3d orbitals. So we will investigate the comparisons related to those 3d orbitals:
• We see that:
(i) The energy of 3d is comparable to the energy of 3s and 3p
(ii) The energy of 3d is again comparable to the energy of 4s and 4p
(iii) The energy of 3d is not comparable to the energy of 4s and 3p
♦ This is because, there is much difference between the energies of those orbitals
✰ The 4s sub-shell is in a 'higher main-shell'
✰ The 3p and 3d sub-shells are in a 'lower main-shell'
3. Based on the above comparison, we can write the 'combinations which are possible' and 'combinations which are not possible'
(i) The following two combinations are possible:
♦ Hybridization involving 3s, 3p and 3d orbitals [based on 2(i)]
✰ This is shown in fig.4.174 below
♦ Hybridization involving 3d, 4s and 4p orbitals [based on 2(ii)]
✰ This is shown in fig.4.175 further below
(ii) The following combination is not possible:
♦ Hybridization involving 3p, 3d and 4s [based on 2(iii)]
✰ This is shown in fig.4.176 further below
✰ The outline is drawn using dashed lines to indicate that, the combination is not possible
 |
| Fig.4.174 |
 |
| Fig.4.175 |
 |
| Fig.4.176 This combination is not possible |
Let us see some real molecules in which, the d orbitals also participates in the hybridization
Structure of PCl5
• When we discussed VSEPR theory, we saw the Lewis dot structure of PCl5
♦ See fig.4.93(c) in section 4.15
♦ All the five P-Cl bonds are single bonds
• For convenience, that fig. is shown again below:
• The details about the shape of PCl5 can be written in 12 steps:
1. The P atom in PCl5 is sp3d hybridized
• Let us see how this sp3d hybridization is achieved. It can be written in 2 steps:
(i) Fig.4.177(a) below shows the orbitals of P
 |
| Fig.4.177 |
• Thus we get five half filled orbitals. This is shown in fig.b
(iii) The 3s orbital mixes together with 3px, 3py, 3pz and 3di. This is also shown in fig.b
(iv) Since there is one s-orbital, three p-orbitals, and one d orbital, it is a sp3d hybridization
2. We know that:
♦ In sp3d hybridization, there will be five resulting hybrid orbitals
• Together, they form a triangular bipyramidal shape
♦ This can be explained in 3 steps:
(i) Fig.4.178(a) below, shows the outlines of a triangular bipyramid
 |
| Fug.4.178 |
✰ The small red sphere is the nucleus of the P atom
✰ This nucleus is situated a the 'center of gravity' of the base triangle
♦ Three green dashed lines radiate out from the nucleus
✰ These lines are at an angular distance of 120o apart
♦ Two red dashed lines radiate upwards and downwards from the nucleus
✰ These lines together form the axis of the bipyramid
♦ The magenta dashed lines are the sloping edges of the bipyramid
(ii) Lines which are required:
♦ We require the following lines:
✰ The three green dashed lines
✰ The two red dashed lines (these two, together form the axis)
♦ We do not require the following lines:
✰ The six magenta dashed lines
✰ The three blue dashed lines
(iii) The required lines are shown in fig.4.178(b)
♦ Altogether, there are five lines
♦ The five hybrid orbitals will be oriented along these lines
♦ This is shown in fig.c
■ Thus we get the 'sp3d hybridized P atom'
3. Let us write three 'important points to remember' about fig.4.178:
(i) The 'plane of the base triangle' is exactly at the middle of the structure
♦ So this plane is called 'equatorial plane'
(ii) All the three green dashed lines lie on the 'plane of the base triangle'
♦ So the three orbitals along the green lines are called 'equatorial orbitals'
♦ These orbitals are at an angular distance of 120o apart
(iii) The two red dashed lines form the axis of the structure
♦ So the two orbitals along the red lines are called 'axial orbitals'
♦ They are at an angular distance of 180o apart
4. So we can write:
♦ The 'sp3d hybridized P atom' consists of two items:
✰ The five hybrid orbitals
✰ The nucleus (small red sphere)
• In the fig.4.178(c), the 'smaller lobes of hybrid orbitals' are not shown
♦ This is to reduce congestion and thus obtain greater clarity
5. Distribution of electrons in the hybrid orbitals:
• This can be written in 3 steps:
(i) We know that, the sp3d hybrid orbitals are formed from 'one s orbital', 'three p orbitals' and 'one d orbital'
(ii) In our present case of P atom, they are: 'one 3s orbital', 'three 3p orbitals' and 'one 3d orbital'
(iii) Before the hybridization, the orbitals mentioned in (ii) carry a total of five electrons
♦ After hybridization, the orbitals mentioned in (ii) will no longer exist
♦ Then what will happen to the three electrons?
Answer:
• The five electrons will be distributed among the five sp3d hybrid orbitals
♦ So each hybrid orbital will carry one electron
♦ This is indicated by the arrows in fig.4.178(c) above
6. So in fig.4.178(c), we have:
♦ Five half filled hybrid orbitals• Now bonding with Cl atoms can begin
• First we will write some basic details about the Cl atom. It can be written in 2 steps:
(We have already seen the two steps in the previous section. But we will write them again)
(i) The electronic configuration of Cl is: [Ne]3s23p5
• Expanding the valence orbitals, the configuration becomes: [Ne]3s2 3px2 3py2 3pz1
(ii) It is clear that, the 3pz of Cl is half filled
• This 3pz needs one more electron
• We saw it in fig.4.166 in the previous section. It is shown again below:
 |
| Fig.4.166 |
♦ It has two arrows
• The py orbital is shown in green color
♦ It has two arrows
• The pz orbital is shown in blue color
♦ It has only one arrow
• The 3s orbital is represented by the cyan sphere
♦ It has two arrows
7. One Cl atom will come and overlap with each of the five half filled orbitals of the P atom
• So a total of five Cl atoms will be coming
♦ In each of those Cl atoms, it is the pz which enters into bonding
♦ This is shown in fig.4.179(a) below:
 |
| Fig.4.179 |
8. The electron clouds of Cl:
(i) The red, green, blue and cyan regions around the Cl nucleus are mere electron clouds
♦ To define the shape of a molecule:
✰ We do not consider the positions of electrons or electron clouds
✰ We consider only the positions of 'nuclei of atoms' in that molecule
(ii) So we do not need to show the orbitals (electron clouds) of the Cl atoms. We can delete them
• However, we will retain the pz orbital. This will convey the fact that, it is 'a p orbital of Cl' that bonds with the P atom
(iii) The final structure is shown in fig.4.179(b) above
• The small yellow spheres represent the nuclei of Cl atoms
• Let us write the salient features of this model. It can be written in 3 steps:
(i) There is a total of five P-Cl bonds
♦ Three of them lie on the equatorial plane
♦ The remaining two lie along the 'axis of the bipyramid'
(ii) So there are:
♦ Three equatorial bonds
♦ Two axial bonds
(iii) The angle between
♦ Any two equatorial bonds is 120o
♦ The two axial bonds is 180o
♦ Any equatorial bond and axial bond is 90o
10. Now we will see an interesting feature about bond lengths
(i) Consider fig.4.178(b) that we saw earlier
♦ There is a total of 5 dashed lines
♦ Those five lines give us the directions of the bonds
(ii) When the PCl5 molecule is formed, each of those five lines will contain a bond pair of electrons
♦ Repulsion will occur between those electron pairs
♦ We can mark those repulsions in the fig.4.178(b)
♦ Fig.4.180(a) below, is one such fig.
✰ It shows the repulsion in the equatorial plane (horizontal plane)
♦ Fig.4.180(b) is another such fig.
✰ It shows the repulsion in the vertical planes
 |
| Fig.4.180 |
• Because, the red double headed arrows will cancel each other out
(iv) But we cannot ignore fig.b
• Let us write the reason. It can be written in three steps (a), (b) and (c):
(a) There are three blue arrows above the equatorial plane
✰ Those arrows will push the 'top most electron pair' away from the equatorial plane
✰ So that electron pair will move further upwards
✰ As a result, the 'bond length' of the 'upper axial bond' will increase
(b) There are three blue arrows below the equatorial plane
✰ Those arrows will push the 'bottom most electron pair' away from the equatorial plane
✰ So that electron pair will move further downwards
✰ As a result, the 'bond length' of the 'lower axial bond' will increase
(c) The difference in bond lengths is shown in fig.4.180(c) above
11. Fig.4.180(c) shows the 2D representation also
• Recall that, we saw the same 2D representation when we analysed PCl5 using VSEPR theory. See fig.4.93(c) in section 4.15
• There we saw the reason for giving the solid triangle, dashed triangle etc.,
12. Note that, all the bonds in PCl5 are sigma bonds
Structure of PF5
1. We saw the trigonal bipyramidal structure of PCl5♦ PF5 also has the same trigonal bipyramidal structure
♦ That means, PF5 will have the same structure as shown in fig.4.179(b) above
2. Note that Cl and F belongs to the same group in the periodic table
♦ The electronic configuration of Cl is [Ne]3s2 3px2 3py2 3pz1
♦ The electronic configuration of F is [He]2s2 2px2 2py2 2pz1
3. Now the reader can write the necessary steps to arrive at the structure of PF5
[Hint: In the case of PF5, the blue orbitals in fig.4.179(b) will be 2pz of F atoms]
• In the next section, we will see the structure of SF6 (Sulfur hexaflouride)

No comments:
Post a Comment