• In the previous section 4.28, we saw the structure of NH3 and H2O. In this section, we will see the structure of BCl3 (Trichloro boron) and BeCl2 (Beryllium chloride)
Structure of BCl3
• Fig.4.164(a) below shows the Lewis dot structure of BCl3
• The details about the model of BCl3 can be written in 10 steps:
1. The B atom in BCl3 is sp2 hybridized
• Let us see how this sp2 hybridization is achieved. It can be written in 2 steps:
(i) Fig.4.164(b) below shows the orbitals in the valence shell of B
(ii) When enough energy is given, one electron in the 2s orbital jumps to the 2py orbital
• Thus we get three half filled orbitals. This is shown in fig.c
(iii) The 2s orbital mixes together with 2px and 2py. This is shown in fig.d
(iv) Since there is one s-orbital and two p-orbitals, it is a sp2 hybridization
2. We know that:
♦ In sp2 hybridization, there will be three resulting hybrid orbitals
✰ Together, they form a triangular planar shape
✰ The larger lobes will be directed towards the corners of a triangle
• This is shown in fig.4.165 below:
• Let us write four 'important points to remember' about fig.4.165:
(i) The three sp2 hybrid orbitals are at an angular distance of 120o apart
(ii) The nucleus of the B atom is shown as a small red sphere
♦ This sphere is situated at the origin of the coordinate axes
(iii) One of the orbitals lies exactly along the x-axis (red axis)
(iv) We know that, all the three sp2 hybrid orbitals will lie on a plane
♦ So in fig.4.165, all the three orbitals are lying on the xy-plane
3. So we can write:
♦ The 'sp2 hybridized B atom' consists of two items:
✰ The three hybrid orbitals
✰ The nucleus (small red sphere)
• In the fig.4.165, the 'smaller lobes of hybrid orbitals' are not shown
♦ This is to reduce congestion and thus obtain greater clarity
4. Distribution of electrons in the hybrid orbitals:
• This can be written in 3 steps:
(i) We know that, the sp2 hybrid orbitals are formed from 'one s orbital' and 'two p orbitals'
(ii) In our present case of B atom, they are: 'one 2s orbital' and 'two 2p orbitals'
(iii) Before the hybridization, the orbitals mentioned in (ii) carry a total of three electrons
♦ After hybridization, the orbitals mentioned in (ii) will no longer exist
♦ Then what will happen to the three electrons?
Answer:
• The three electrons will be distributed among the three sp2 hybrid orbitals
♦ So each hybrid orbital will carry one electron
♦ This is indicated by the arrows in fig.4.165 above
• Now bonding with Cl atoms can begin
• First we will write some basic details about the Cl atom. it can be written in 2 steps:
(i) The electronic configuration of Cl is: [Ne]3s23p5
• Expanding the valence orbitals, the configuration becomes: [Ne]3s2 3px2 3py2 3pz1
(ii) It is clear that, the 3pz of Cl is half filled
• This 3pz needs one more electron
• This is shown in fig.4.166 below:
• The px orbital is shown in red color
♦ It has two arrows
• The py orbital is shown in green color
♦ It has two arrows
• The pz orbital is shown in blue color
♦ It has only one arrow
• The 3s orbital is represented by the cyan sphere
♦ It has two arrows
6. One Cl atom will come and overlap with each of the three half filled orbitals of the B atom
• So a total of three Cl atoms will be coming
♦ In each of those Cl atoms, it is the pz which enters into bonding
♦ This is shown in fig.4.167 below:
• In the fig.4.167 above, the 'pz orbitals of Cl' have become completely filled
7. Is the structure 3D or 2D?
The answer can be written in 5 steps:
(i) The structure in the above fig.4.167, appears to be a 3D structure
• Because, the red orbitals of the Cl atoms are protruding above and below the xy-plane
(ii) But the red orbitals are mere electron clouds
♦ To define the shape of a molecule:
✰ We do not consider the positions of electrons or electron clouds
✰ We consider only the positions of 'nuclei of atoms' in that molecule
(iii) We see that, all the three Cl nuclei lie in the same plane as the nucleus of B
(Remember that, the nucleus of a Cl atom is at the center of the cyan sphere)
(iv) So the structure is planar. In other words, it is a 2D structure
• We do not need to show the orbitals (electron clouds) of the Cl atoms. So we can delete them
• However, we will retain the pz orbital. This will convey the fact that, it is 'a p orbital of Cl' that bonds with the B atom
(v) The final structure is shown in fig.4.168(a) below.
• The small yellow spheres represent the nuclei of Cl atoms
8. Thus we get the model of the BCl3 molecule
• Let us write the salient features of this model. It can be written in 3 steps:
(i) The three Cl atoms are situated at the three corners of a triangle
(ii) The B atom is situated at the 'center of gravity' of the triangle
(iii) The angle between any two bonds is 120o
9. The 2D representation of the BCl3 molecule is shown on fig.4.168(b) above
10. Note that, all the bonds in BCl3 are sigma bonds
Structure of BeCl2
• Fig.4.169(a) below shows the Lewis dot structure of BeCl2
• The details about the model of BeCl2 can be written in 10 steps:
1. The Be atom in BeCl2 is sp hybridized
• Let us see how this sp hybridization is achieved. It can be written in 2 steps:
(i) Fig.4.169(b) below shows the orbitals in the valence shell of Be
(ii) When enough energy is given, one electron in the 2s orbital jumps to the 2px orbital
• Thus we get two half filled orbitals. This is shown in fig.c
(iii) The 2s orbital mixes together with 2px. This is shown in fig.d
(iv) Since there is one s-orbital and one p-orbital, it is a sp hybridization
2. We know that:
♦ In sp hybridization, there will be two resulting hybrid orbitals
✰ Together, they form a linear shape
• This is shown in fig.4.170 below:
• Let us write four 'important points to remember' about fig.4.170:
(i) The two sp hybrid orbitals are at an angular distance of 180o apart
(ii) The nucleus of the Be atom is shown as a small red sphere
♦ This sphere is situated at the origin of the coordinate axes
(iii) Both the orbitals lie exactly along the x-axis (red axis)
(iv) We know that, each hybrid orbital have a larger lobe and a smaller lobe
• In the previous cases (except C2H2), we deliberately chose not to show the smaller lobes. This was for better clarity
• In our present case, we do not have to hide the smaller lobes because, they are automatically hidden
♦ The smaller lobe of the left side orbital is inside the larger lobe of the right side orbital
♦ The smaller lobe of the right side orbital is inside the larger lobe of the left side orbital
(Recall that, in C2H2 also, we encountered the same situation)
3. So we can write:
♦ The 'sp hybridized B atom' consists of two items:
✰ The two hybrid orbitals
✰ The nucleus (small red sphere)
4. Distribution of electrons in the hybrid orbitals:
• This can be written in 3 steps:
(i) We know that, the sp hybrid orbitals are formed from 'one s orbital' and 'one p orbital'
(ii) In our present case of Be atom, they are: 'one 2s orbital' and 'one 2p orbital'
(iii) Before the hybridization, the orbitals mentioned in (ii) carry a total of two electrons
♦ After hybridization, the orbitals mentioned in (ii) will no longer exist
♦ Then what will happen to the two electrons?
Answer:
• The two electrons will be distributed among the two sp hybrid orbitals
♦ So each hybrid orbital will carry one electron
♦ This is indicated by the arrows in fig.4.170 above
• Now bonding with Cl atoms can begin
• First we will write some basic details about the Cl atom. it can be written in 2 steps:
(We have already seen the two steps when we discussed BCl3 above. But we will write them again)
(i) The electronic configuration of Cl is: [Ne]3s23p5
• Expanding the valence orbitals, the configuration becomes: [Ne]3s2 3px2 3py2 3pz1
(ii) It is clear that, the 3pz of Cl is half filled
• This 3pz needs one more electron. This was shown in fig.4.166 above
• The px orbital is shown in red color
♦ It has two arrows
• The py orbital is shown in green color
♦ It has two arrows
• The pz orbital is shown in blue color
♦ It has only one arrow
• The 3s orbital is represented by the cyan sphere
♦ It has two arrows
6. One Cl atom will come and overlap with each of the two half filled orbitals of the Be atom
• So a total of two Cl atoms will be coming
♦ In each of those Cl atoms, it is the pz which enters into bonding
♦ This is shown in fig.4.171 below:
• In the fig.4.171 above, the 'pz orbitals of Cl' have become completely filled
7. Is the structure 3D or 2D?
The answer can be written in 5 steps:
(i) The structure in the above fig.4.171, appears to be a 3D structure
• Because, the red orbitals of the Cl atoms are protruding above and below the xy-plane
(ii) But the red orbitals are mere electron clouds
♦ To define the shape of a molecule:
✰ We do not consider the positions of electrons or electron clouds
✰ We consider only the positions of 'nuclei of atoms' in that molecule
(iii) We see that, all the three Cl nuclei lie in the same plane as the nucleus of B
(Remember that, the nucleus of a Cl atom is at the center of the cyan sphere)
(iv) So the structure is planar. In other words, it is a 2D structure
• We do not need to show the orbitals (electron clouds) of the Cl atoms. So we can delete them
• However, we will retain the pz orbital. This will convey the fact that, it is 'a p orbital of Cl' that bonds with the Be atom
(v) The final structure is shown in fig.4.172(a) below.
• The small yellow spheres represent the nuclei of Cl atoms
8. Thus we get the model of the BeCl2 molecule
• Let us write the salient features of this model. It can be written in 4 steps:
(i) The two Cl atoms are situated at the opposite ends of a line
(ii) The Be atom is situated at the 'center of gravity' (midpoint) of the line
(iii) The angle between the two bonds is 180o
(iv) So it is a linear structure
9. The 2D representation of the BeCl2 molecule is shown on fig.4.172(b) above
10. Note that, both the bonds in BeCl2 are sigma bonds
Structure of BCl3
• Fig.4.164(a) below shows the Lewis dot structure of BCl3
• The details about the model of BCl3 can be written in 10 steps:
1. The B atom in BCl3 is sp2 hybridized
• Let us see how this sp2 hybridization is achieved. It can be written in 2 steps:
(i) Fig.4.164(b) below shows the orbitals in the valence shell of B
 |
| Fig.4.164 |
• Thus we get three half filled orbitals. This is shown in fig.c
(iii) The 2s orbital mixes together with 2px and 2py. This is shown in fig.d
(iv) Since there is one s-orbital and two p-orbitals, it is a sp2 hybridization
2. We know that:
♦ In sp2 hybridization, there will be three resulting hybrid orbitals
✰ Together, they form a triangular planar shape
✰ The larger lobes will be directed towards the corners of a triangle
• This is shown in fig.4.165 below:
 |
| Fig.4.165 |
(i) The three sp2 hybrid orbitals are at an angular distance of 120o apart
(ii) The nucleus of the B atom is shown as a small red sphere
♦ This sphere is situated at the origin of the coordinate axes
(iii) One of the orbitals lies exactly along the x-axis (red axis)
(iv) We know that, all the three sp2 hybrid orbitals will lie on a plane
♦ So in fig.4.165, all the three orbitals are lying on the xy-plane
3. So we can write:
♦ The 'sp2 hybridized B atom' consists of two items:
✰ The three hybrid orbitals
✰ The nucleus (small red sphere)
• In the fig.4.165, the 'smaller lobes of hybrid orbitals' are not shown
♦ This is to reduce congestion and thus obtain greater clarity
4. Distribution of electrons in the hybrid orbitals:
• This can be written in 3 steps:
(i) We know that, the sp2 hybrid orbitals are formed from 'one s orbital' and 'two p orbitals'
(ii) In our present case of B atom, they are: 'one 2s orbital' and 'two 2p orbitals'
(iii) Before the hybridization, the orbitals mentioned in (ii) carry a total of three electrons
♦ After hybridization, the orbitals mentioned in (ii) will no longer exist
♦ Then what will happen to the three electrons?
Answer:
• The three electrons will be distributed among the three sp2 hybrid orbitals
♦ So each hybrid orbital will carry one electron
♦ This is indicated by the arrows in fig.4.165 above
5. So in fig.4.165, we have:
♦ Three half filled hybrid orbitals• Now bonding with Cl atoms can begin
• First we will write some basic details about the Cl atom. it can be written in 2 steps:
(i) The electronic configuration of Cl is: [Ne]3s23p5
• Expanding the valence orbitals, the configuration becomes: [Ne]3s2 3px2 3py2 3pz1
(ii) It is clear that, the 3pz of Cl is half filled
• This 3pz needs one more electron
• This is shown in fig.4.166 below:
 |
| Fig.4.166 |
♦ It has two arrows
• The py orbital is shown in green color
♦ It has two arrows
• The pz orbital is shown in blue color
♦ It has only one arrow
• The 3s orbital is represented by the cyan sphere
♦ It has two arrows
6. One Cl atom will come and overlap with each of the three half filled orbitals of the B atom
• So a total of three Cl atoms will be coming
♦ In each of those Cl atoms, it is the pz which enters into bonding
♦ This is shown in fig.4.167 below:
 |
| Fig.4.167 |
7. Is the structure 3D or 2D?
The answer can be written in 5 steps:
(i) The structure in the above fig.4.167, appears to be a 3D structure
• Because, the red orbitals of the Cl atoms are protruding above and below the xy-plane
(ii) But the red orbitals are mere electron clouds
♦ To define the shape of a molecule:
✰ We do not consider the positions of electrons or electron clouds
✰ We consider only the positions of 'nuclei of atoms' in that molecule
(iii) We see that, all the three Cl nuclei lie in the same plane as the nucleus of B
(Remember that, the nucleus of a Cl atom is at the center of the cyan sphere)
(iv) So the structure is planar. In other words, it is a 2D structure
• We do not need to show the orbitals (electron clouds) of the Cl atoms. So we can delete them
• However, we will retain the pz orbital. This will convey the fact that, it is 'a p orbital of Cl' that bonds with the B atom
(v) The final structure is shown in fig.4.168(a) below.
• The small yellow spheres represent the nuclei of Cl atoms
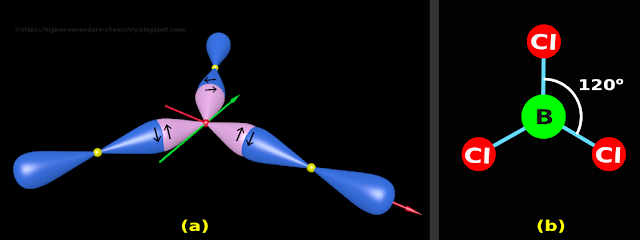 |
| Fig.4.168 |
8. Thus we get the model of the BCl3 molecule
• Let us write the salient features of this model. It can be written in 3 steps:
(i) The three Cl atoms are situated at the three corners of a triangle
(ii) The B atom is situated at the 'center of gravity' of the triangle
(iii) The angle between any two bonds is 120o
9. The 2D representation of the BCl3 molecule is shown on fig.4.168(b) above
10. Note that, all the bonds in BCl3 are sigma bonds
Structure of BeCl2
• Fig.4.169(a) below shows the Lewis dot structure of BeCl2
• The details about the model of BeCl2 can be written in 10 steps:
1. The Be atom in BeCl2 is sp hybridized
• Let us see how this sp hybridization is achieved. It can be written in 2 steps:
(i) Fig.4.169(b) below shows the orbitals in the valence shell of Be
 |
| Fig.4.169 |
• Thus we get two half filled orbitals. This is shown in fig.c
(iii) The 2s orbital mixes together with 2px. This is shown in fig.d
(iv) Since there is one s-orbital and one p-orbital, it is a sp hybridization
2. We know that:
♦ In sp hybridization, there will be two resulting hybrid orbitals
✰ Together, they form a linear shape
• This is shown in fig.4.170 below:
 |
| Fig.4.170 |
(i) The two sp hybrid orbitals are at an angular distance of 180o apart
(ii) The nucleus of the Be atom is shown as a small red sphere
♦ This sphere is situated at the origin of the coordinate axes
(iii) Both the orbitals lie exactly along the x-axis (red axis)
(iv) We know that, each hybrid orbital have a larger lobe and a smaller lobe
• In the previous cases (except C2H2), we deliberately chose not to show the smaller lobes. This was for better clarity
• In our present case, we do not have to hide the smaller lobes because, they are automatically hidden
♦ The smaller lobe of the left side orbital is inside the larger lobe of the right side orbital
♦ The smaller lobe of the right side orbital is inside the larger lobe of the left side orbital
(Recall that, in C2H2 also, we encountered the same situation)
3. So we can write:
♦ The 'sp hybridized B atom' consists of two items:
✰ The two hybrid orbitals
✰ The nucleus (small red sphere)
4. Distribution of electrons in the hybrid orbitals:
• This can be written in 3 steps:
(i) We know that, the sp hybrid orbitals are formed from 'one s orbital' and 'one p orbital'
(ii) In our present case of Be atom, they are: 'one 2s orbital' and 'one 2p orbital'
(iii) Before the hybridization, the orbitals mentioned in (ii) carry a total of two electrons
♦ After hybridization, the orbitals mentioned in (ii) will no longer exist
♦ Then what will happen to the two electrons?
Answer:
• The two electrons will be distributed among the two sp hybrid orbitals
♦ So each hybrid orbital will carry one electron
♦ This is indicated by the arrows in fig.4.170 above
5. So in fig.4.170, we have:
♦ Two half filled hybrid orbitals• Now bonding with Cl atoms can begin
• First we will write some basic details about the Cl atom. it can be written in 2 steps:
(We have already seen the two steps when we discussed BCl3 above. But we will write them again)
(i) The electronic configuration of Cl is: [Ne]3s23p5
• Expanding the valence orbitals, the configuration becomes: [Ne]3s2 3px2 3py2 3pz1
(ii) It is clear that, the 3pz of Cl is half filled
• This 3pz needs one more electron. This was shown in fig.4.166 above
♦ It has two arrows
• The py orbital is shown in green color
♦ It has two arrows
• The pz orbital is shown in blue color
♦ It has only one arrow
• The 3s orbital is represented by the cyan sphere
♦ It has two arrows
6. One Cl atom will come and overlap with each of the two half filled orbitals of the Be atom
• So a total of two Cl atoms will be coming
♦ In each of those Cl atoms, it is the pz which enters into bonding
♦ This is shown in fig.4.171 below:
 |
| Fig.4.171 |
7. Is the structure 3D or 2D?
The answer can be written in 5 steps:
(i) The structure in the above fig.4.171, appears to be a 3D structure
• Because, the red orbitals of the Cl atoms are protruding above and below the xy-plane
(ii) But the red orbitals are mere electron clouds
♦ To define the shape of a molecule:
✰ We do not consider the positions of electrons or electron clouds
✰ We consider only the positions of 'nuclei of atoms' in that molecule
(iii) We see that, all the three Cl nuclei lie in the same plane as the nucleus of B
(Remember that, the nucleus of a Cl atom is at the center of the cyan sphere)
(iv) So the structure is planar. In other words, it is a 2D structure
• We do not need to show the orbitals (electron clouds) of the Cl atoms. So we can delete them
• However, we will retain the pz orbital. This will convey the fact that, it is 'a p orbital of Cl' that bonds with the Be atom
(v) The final structure is shown in fig.4.172(a) below.
• The small yellow spheres represent the nuclei of Cl atoms
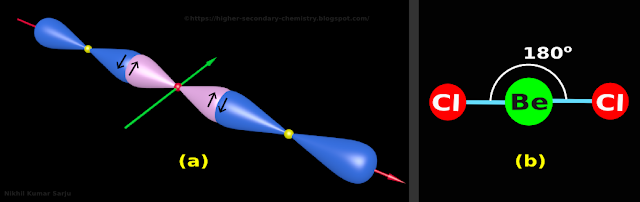 |
| Fig.4.172 |
8. Thus we get the model of the BeCl2 molecule
• Let us write the salient features of this model. It can be written in 4 steps:
(i) The two Cl atoms are situated at the opposite ends of a line
(ii) The Be atom is situated at the 'center of gravity' (midpoint) of the line
(iii) The angle between the two bonds is 180o
(iv) So it is a linear structure
9. The 2D representation of the BeCl2 molecule is shown on fig.4.172(b) above
10. Note that, both the bonds in BeCl2 are sigma bonds
• In the next section, we will see the hybridization involving d orbitals
No comments:
Post a Comment