In the previous section 4.26, we saw the structure of C2H4. We also discussed pi bond. In this section, we will see the structure of C2H2 (ethyne)
• Fig.4.153(a) below shows the Lewis dot structure of C2H2
• The details about the model of C2H2 can be written in 13 steps:
1. The C atom in C2H2 is sp hybridized
• Let us see how this sp hybridization is achieved. It can be written in 4 steps:
(i) Fig.4.153(b) below shows the orbitals in the valence shell of C
(ii) When enough energy is given, one electron in the 2s orbital jumps to the 2pz orbital
• Thus we get four half filled orbitals. This is shown in fig.c
(iii) The 2s orbital mixes together with 2px. This is shown in fig.d
(Note that, 2py and 2pz do not participate in the mixing)
(iv) Since there is one s-orbital and one p-orbital, it is a sp hybridization
2. We know that:
♦ In sp hybridization, there will be two hybrid orbitals
✰ Together, they form a linear shape
• This is shown in fig.4.154(a) below:
• Let us write four 'important points to remember' about fig.a:
(i) The two sp hybrid orbitals are at an angular distance of 180o apart
(ii) The nucleus of the C atom is shown as a small red sphere
♦ This sphere is situated at the origin of the coordinate axes
(iii) Both the orbitals lie exactly along the x-axis (red axis)
(iv) We know that, each hybrid orbital have a larger lobe and a smaller lobe
• In the previous two sections, we deliberately chose not to show the smaller lobes. This was for better clarity
• In our present case, we do not have to hide the smaller lobes because, they are automatically hidden
♦ The smaller lobe of the left side orbital is inside the larger lobe of the right side orbital
♦ The smaller lobe of the right side orbital is inside the larger lobe of the left side orbital
3. Remember that, the 2py and 2pz orbitals did not take part in the hybridization
♦ So they will 'remain as such' even after hybridization
♦ We have to show them in fig.4.154(a)
• The modified model is shown in fig.b
• This model in fig.b represents the 'sp hybridized C atom'
• So we can write:
♦ The 'sp hybridized C atom' consists of four items:
✰ The two hybrid orbitals
✰ The 2py orbital
✰ The 2pz orbital
✰ The nucleus (small red sphere)
4. Distribution of electrons in the hybrid orbitals:
• This can be written in 3 steps:
(i) We know that, the sp hybrid orbitals are formed from 'one s orbital' and 'one p orbital'
(ii) In our present case of C atom, they are: 'one 2s orbital' and 'one 2p orbital'
(iii) Before the hybridization, the orbitals mentioned in (ii) carry a total of two electrons
♦ After hybridization, the orbitals mentioned in (ii) will no longer exist
♦ Then what will happen to the two electrons?
Answer:
• The two electrons will be distributed among the two sp hybrid orbitals
♦ So each hybrid orbital will carry one electron
♦ This is indicated by the arrows in fig.4.154(a) above
5. Remember that,
♦ the 2py orbital also has one electron
♦ the 2pz orbital also has one electron
• So an arrow is shown
♦ in the 2py orbital in fig.b also
♦ in the 2pz orbital in fig.b also
6. So we have a 'sp hybridized C atom' in fig.4.154(b)
• This C atom is
♦ symmetrical along the x-axis
♦ symmetrical about the nucleus
• Since there is symmetry, we do not want a 'mirror image' of that C atom
♦ An 'exact copy' is what we want
• The 'copy' is shown in the fig.4.155(a) below
7. So now we have two C atoms
• Remember that, each one of them is sp hybridized
• We want the two C atoms to bond together
• For that we use the following four steps:
(i) We have two orbitals lying along the x-axis
♦ One belongs to the 'original C atom'
♦ The other belongs to the 'copy C atom'
(ii) Move the 'copy' towards the original
• The movement should be exactly along the x-axis
(iii) When an optimum distance is reached, overlapping takes place between the 'two orbitals mentioned in (i)'
♦ This is shown in fig.4.155(b) above
• 'overlapping' means that, there will be a particular region, which will belong to both the orbitals
• This region is called 'overlapping region'
(iv) The two electrons (one from each orbital) will then lie in that 'overlapping region'
• Thus the two electrons will belong to both the C atoms
• In this way, a bond is formed between those two C atoms
• This is shown in fig.4.156(a) below:
8. So in fig.4.156(a) above, we have two C atoms which are bonded together
• Now bonding with H atoms can begin:
♦ One H atom will come and overlap with the free orbital of the original C atom
♦ Another H atom will come and overlap with the free orbital of the 'C atom which is the copy'
♦ This is shown in fig.4.156(b)
9. The structure in fig.4.156(b), is a single unit. It consists of:
♦ Two C atoms
♦ Two H atoms
• But the molecule of C2H2 is not yet formed. This is because:
♦ each of the two py orbitals is still half occupied
♦ each of the two pz orbitals is still half occupied
10. Each of the two py orbitals in fig.4.156(b) above needs one more electron
• Each of the two pz orbitals in fig.4.156(b) above also needs one more electron
• So they share electrons among themselves
• We have already seen how the sharing takes place. We saw it in the case of C2H4 in the previous section
♦ The two py clouds overlap in a 'side-wise manner'
` ✰ The result is the formation of two 'U' shaped clouds in the y direction
♦ The two pz clouds overlap in a 'side-wise manner'
✰ The result is the formation of two 'U' shaped clouds in the z direction
11. So the final structure of the C2H2 molecule will be as shown in fig.4.157(a) below:
• A break up is shown in fig.4.157(b)
♦ The 'U' shaped clouds are shown separated
♦ This will help us to get a clearer idea about the structure
12. 2D representation of the molecule:
• It is obvious that, both the C atoms and both the H atoms will lie on a plane
• So the 2D representation of C2H2 will be as shown in the fig.4.158 below
♦ It will not have any solid triangles
♦ It will not have any dashed triangles
♦ It will have only solid lines
• Note that, C2H2 has a linear shape
13. Next we will see an interesting point. It can be written in 3 steps:
(i) Consider the final model in fig.4.157 above
• Let us consider it as two separate units
♦ The original C atom and it's H atom constitute the first unit
♦ The copy C atom and it's H atom constitute the second unit
(ii) Keep the first unit 'fixed'
♦ Rotate the second unit
♦ Nucleus of the second unit is the pivot of rotation
♦ x-axis is the axis of rotation
• This is indicated by the yellow curved arrow in fig.4.159 below
(iii) We can do this type of rotation, only by causing damage to all the 'U' shaped orbitals
• We saw the 'type of damage' in fig.4.151 of the previous section
• So such a rotation is not possible
• So we will apply it directly to our present case of C2H2. It can be explained in 2 steps:
1. It is obvious that, there are two pi bonds between the C atoms in C2H2
• They are:
♦ The magenta 'U' shaped orbitals
♦ The yellow 'U' shaped orbitals
2. In the Lewis structure of C2H2, we see a triple bond between the two C atoms
(See fig.4.153(a) at the beginning of this section)
• A triple bond is represented by putting a '≡' between atoms
• So now we know that:
♦ the upper '-' in the '≡' is a 𝛔 bond
♦ the middle '-' in the '≡' is a 𝛑 bond
♦ the lower '-' in the '≡' is a 𝛑 bond
• Fig.4.153(a) below shows the Lewis dot structure of C2H2
• The details about the model of C2H2 can be written in 13 steps:
1. The C atom in C2H2 is sp hybridized
• Let us see how this sp hybridization is achieved. It can be written in 4 steps:
(i) Fig.4.153(b) below shows the orbitals in the valence shell of C
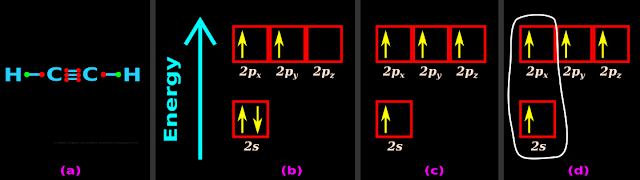 |
| Fig.4.153 |
• Thus we get four half filled orbitals. This is shown in fig.c
(iii) The 2s orbital mixes together with 2px. This is shown in fig.d
(Note that, 2py and 2pz do not participate in the mixing)
(iv) Since there is one s-orbital and one p-orbital, it is a sp hybridization
2. We know that:
♦ In sp hybridization, there will be two hybrid orbitals
✰ Together, they form a linear shape
• This is shown in fig.4.154(a) below:
 |
| Fig.4.154 |
(i) The two sp hybrid orbitals are at an angular distance of 180o apart
(ii) The nucleus of the C atom is shown as a small red sphere
♦ This sphere is situated at the origin of the coordinate axes
(iii) Both the orbitals lie exactly along the x-axis (red axis)
(iv) We know that, each hybrid orbital have a larger lobe and a smaller lobe
• In the previous two sections, we deliberately chose not to show the smaller lobes. This was for better clarity
• In our present case, we do not have to hide the smaller lobes because, they are automatically hidden
♦ The smaller lobe of the left side orbital is inside the larger lobe of the right side orbital
♦ The smaller lobe of the right side orbital is inside the larger lobe of the left side orbital
3. Remember that, the 2py and 2pz orbitals did not take part in the hybridization
♦ So they will 'remain as such' even after hybridization
♦ We have to show them in fig.4.154(a)
• The modified model is shown in fig.b
• This model in fig.b represents the 'sp hybridized C atom'
• So we can write:
♦ The 'sp hybridized C atom' consists of four items:
✰ The two hybrid orbitals
✰ The 2py orbital
✰ The 2pz orbital
✰ The nucleus (small red sphere)
4. Distribution of electrons in the hybrid orbitals:
• This can be written in 3 steps:
(i) We know that, the sp hybrid orbitals are formed from 'one s orbital' and 'one p orbital'
(ii) In our present case of C atom, they are: 'one 2s orbital' and 'one 2p orbital'
(iii) Before the hybridization, the orbitals mentioned in (ii) carry a total of two electrons
♦ After hybridization, the orbitals mentioned in (ii) will no longer exist
♦ Then what will happen to the two electrons?
Answer:
• The two electrons will be distributed among the two sp hybrid orbitals
♦ So each hybrid orbital will carry one electron
♦ This is indicated by the arrows in fig.4.154(a) above
5. Remember that,
♦ the 2py orbital also has one electron
♦ the 2pz orbital also has one electron
• So an arrow is shown
♦ in the 2py orbital in fig.b also
♦ in the 2pz orbital in fig.b also
6. So we have a 'sp hybridized C atom' in fig.4.154(b)
• This C atom is
♦ symmetrical along the x-axis
♦ symmetrical about the nucleus
• Since there is symmetry, we do not want a 'mirror image' of that C atom
♦ An 'exact copy' is what we want
• The 'copy' is shown in the fig.4.155(a) below
 |
| Fig.4.155 |
• Remember that, each one of them is sp hybridized
• We want the two C atoms to bond together
• For that we use the following four steps:
(i) We have two orbitals lying along the x-axis
♦ One belongs to the 'original C atom'
♦ The other belongs to the 'copy C atom'
(ii) Move the 'copy' towards the original
• The movement should be exactly along the x-axis
(iii) When an optimum distance is reached, overlapping takes place between the 'two orbitals mentioned in (i)'
♦ This is shown in fig.4.155(b) above
• 'overlapping' means that, there will be a particular region, which will belong to both the orbitals
• This region is called 'overlapping region'
(iv) The two electrons (one from each orbital) will then lie in that 'overlapping region'
• Thus the two electrons will belong to both the C atoms
• In this way, a bond is formed between those two C atoms
• This is shown in fig.4.156(a) below:
 |
| Fig.4.156 |
• Now bonding with H atoms can begin:
♦ One H atom will come and overlap with the free orbital of the original C atom
♦ Another H atom will come and overlap with the free orbital of the 'C atom which is the copy'
♦ This is shown in fig.4.156(b)
9. The structure in fig.4.156(b), is a single unit. It consists of:
♦ Two C atoms
♦ Two H atoms
• But the molecule of C2H2 is not yet formed. This is because:
♦ each of the two py orbitals is still half occupied
♦ each of the two pz orbitals is still half occupied
10. Each of the two py orbitals in fig.4.156(b) above needs one more electron
• Each of the two pz orbitals in fig.4.156(b) above also needs one more electron
• So they share electrons among themselves
• We have already seen how the sharing takes place. We saw it in the case of C2H4 in the previous section
♦ The two py clouds overlap in a 'side-wise manner'
` ✰ The result is the formation of two 'U' shaped clouds in the y direction
♦ The two pz clouds overlap in a 'side-wise manner'
✰ The result is the formation of two 'U' shaped clouds in the z direction
11. So the final structure of the C2H2 molecule will be as shown in fig.4.157(a) below:
 |
| Fig.4.157 |
♦ The 'U' shaped clouds are shown separated
♦ This will help us to get a clearer idea about the structure
12. 2D representation of the molecule:
• It is obvious that, both the C atoms and both the H atoms will lie on a plane
 |
| Fig.4.158 |
♦ It will not have any dashed triangles
♦ It will have only solid lines
• Note that, C2H2 has a linear shape
13. Next we will see an interesting point. It can be written in 3 steps:
(i) Consider the final model in fig.4.157 above
• Let us consider it as two separate units
♦ The original C atom and it's H atom constitute the first unit
♦ The copy C atom and it's H atom constitute the second unit
(ii) Keep the first unit 'fixed'
♦ Rotate the second unit
♦ Nucleus of the second unit is the pivot of rotation
♦ x-axis is the axis of rotation
• This is indicated by the yellow curved arrow in fig.4.159 below
 |
| Fig.4.159 |
• We saw the 'type of damage' in fig.4.151 of the previous section
• In the previous section, the structure of C2H4 gives us an opportunity to learn about pi bond
1. It is obvious that, there are two pi bonds between the C atoms in C2H2
• They are:
♦ The magenta 'U' shaped orbitals
♦ The yellow 'U' shaped orbitals
2. In the Lewis structure of C2H2, we see a triple bond between the two C atoms
(See fig.4.153(a) at the beginning of this section)
• A triple bond is represented by putting a '≡' between atoms
• So now we know that:
♦ the upper '-' in the '≡' is a 𝛔 bond
♦ the middle '-' in the '≡' is a 𝛑 bond
♦ the lower '-' in the '≡' is a 𝛑 bond
• In the next section, we will see the structure of NH3 (ammonia)
No comments:
Post a Comment