In the previous section, we saw that, benzene has unusual stability. In this section, we will see the explanation for such a stability.
The explanation can be written in 12 steps:
1. Each of the six C atoms in benzene is sp2 hybridized. So each of them will have three hybrid orbitals.
2. Consider any one C atom.
•
It can use two hybrid orbitals to form 𝜎 bonds with two adjacent C atoms. Such 𝜎 bonds are shown in red color in fig.13.99 below:
 |
| Fig.13.99 |
•
The remaining hybrid orbital can be used to form a 𝜎 bond with the s-orbital of a H atom. Such 𝜎 bonds are shown in green color in the fig.13.99.
•
For example:
♦ The C3 forms 𝜎 bonds with C2 and C4
♦ This C3 atom forms 𝜎 bond with a H atom also.
•
All the 𝜎 bonds lie on a hexagonal plane.
3. Recall that, any C atom which is sp2 hybridized, will have an unhybridized p orbital. This p orbital will be perpendicular to the hexagonal plane. Those p orbitals are shown in magenta color in fig.13.99.
4. The p orbitals can overlap in lateral direction to form π bonds.
•
Two p orbitals are required to form one π bond. We have six p orbitals. So there will be three π bonds.
◼ In fig.13.100(a) below,
♦ p orbitals of C1 and C2 overlaps laterally to form a π bond.
♦ p orbitals of C3 and C4 overlaps laterally to form a π bond.
♦ p orbitals of C5 and C6 overlaps laterally to form a π bond.
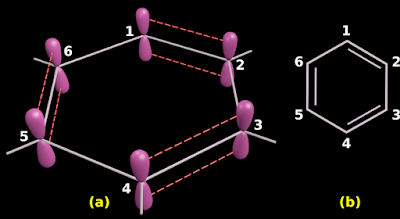 |
| Fig.13.100 |
•
This arrangement will result in the alternate single bonds and double bonds as shown in fig.13.100(b)
◼ In fig.13.101(a) below,
♦ p orbitals of C2 and C3 overlaps laterally to form a π bond.
♦ p orbitals of C4 and C5 overlaps laterally to form a π bond.
♦ p orbitals of C6 and C1 overlaps laterally to form a π bond.
 |
| Fig.13.101 |
•
This arrangement will result in the alternate single bonds and double bonds as shown in fig.13.101(b).
5. Consider the two arrangements:
♦ Fig.13.100(a) shows one possible arrangement.
♦ Fig.13.101(b) shows the second possible arrangement.
•
Both the arrangements are equally likely to occur.
•
That means, at any instant, we will be seeing an electron cloud distributed over the entire area.
•
This is shown in fig.13.102(a) below:
 |
| Fig.13.102 |
6. When the electrons belong to particular C atoms, we say that those electrons are localized.
♦ In fig.100 and 101, the electrons in the p orbitals are localized.
• But when the electrons do not belong to particular C atoms, we say that, those electrons are delocalized.
♦ In fig.102, the electrons in the p orbitals are delocalized.
7. The delocalization as in fig.102 will result in an electron cloud which is distributed over the whole area of the molecule.
• Fig.103 below shows three layers.
(i) The upper red layer is the cloud formed by all the upper lobes of the p orbitals.
(ii) The lower red layer is the cloud formed by all the lower lobes of the p orbitals.
(iii) The middle grey hexagonal layer is the plane in which the six C atoms are situated.
 |
| Fig.13.103 |
8. The three layers act together as a single unit.
•
Such an unit will have complete symmetry.
•
We will not be able to say that:
♦ some portions of the molecule have single bonds.
♦ some other portions have double bonds.
9 . When the electron clouds of the p orbitals are distributed over the entire area, they are better attracted by the nuclei of the C atoms.
•
If the electron clouds are localized between particular C atoms, such a high amount of attraction will not be possible.
•
So the delocalization gives extra stability to the benzene molecule.
10. Let us see some experimental results which can be used as proof for the structure shown in fig.103. It can be written in two steps:
(i) X-ray diffraction data shows that, benzene has a planar structure.
(ii) Imagine that, benzene indeed has alternate single bonds and double bonds. Then:
♦ The distance between some of the adjacent C atoms would be 154 pm
(The C-C single bond has a bond length of 154 pm)
♦ The distance between the remaining of the adjacent C atoms would be 133 pm
(The C=C double bond has a bond length of 133 pm)
•
But X-ray data shows that distance between any two adjacent C atoms in benzene are same, which is 139 pm.
•
Note that, 139 is a value which is intermediate between 133 and 154. This indicates that:
♦ There are no pure single bonds in benzene.
♦ There are no pure double bonds in benzene.
11. The absence of pure double bonds is the reason why benzene cannot undergo addition reactions in normal conditions. The reluctance to addition reactions puzzled scientists because, they expect all molecules containing double bonds to undergo addition reactions. Also, double or triple bonds are necessary to explain how six H atoms are able to satisfy the valencies of six C atoms.
12. It is not convenient to always draw the 3D views showing the details of p orbitals. So we adopt the method shown in fig.104 below:
 |
| Fig.13.104 |
•
The fig.104 shows that, the actual benzene molecule is a hybrid of (A) and (B). The hybrid is shown in (C). Since the π electrons are delocalized, we insert a circle inside the hexagon.
In the next section, we will see it aromaticity.
Copyright©2022 Higher secondary chemistry.blogspot.com
No comments:
Post a Comment