In the previous section,
we saw that, a SE reaction proceeds in three steps:
(a) Generation of the electrophile ($\rm{E^{\oplus}}$)
(b) Formation of carbocation intermediate
(c) Removal of proton from carbocation intermediate
•
We saw the details of the first step. In this section, we will see the remaining two steps.
(b) Formation of carbocation intermediate
This can be written in 6 steps:
1. The electrophile produced in the first step, attacks the benzene ring. This is shown in fig.13.116 below:
 |
| Fig.13.116 |
•
Two electrons in a π bond are transferred to the electrophile. This is indicated by the green curved arrow.
2. Those two electrons are used for forming a bond between the electrophile and the benzene ring. Thus the E gets attached to the ring.
•
But now a double bond is lost.
3. In the fig.13.116 above, in the product side, consider the C atom to which E and H are attached.
•
This C atom is attached to four other atoms:
Two C atoms, One H atom and One E atom.
•
So there are four bonds. They are all single bonds. So they are all σ bonds.
•
C atom can participate in four σ bonds only if it is sp3 hybridized.
So this C atom is sp3 hybridized.
4. In the fig.13.116 above, in the product side, consider the C atom to which a single H atom is attached. There are only three bonds around this C atom.
•
That means there are only three electrons immediately around this C atom.
•
An independent C atom will have four electrons immediately around it. So this C atom will have a +ve formal charge.
5. The product formed in fig.13.116 above is called σ complex. It is also known as arenium ion.
•
The arenium ion gets stabilized by resonance. This is shown in fig.13.117 below:
 |
| Fig.13.117 |
• The +ve charge moves between three C atoms. There is conjugation between those three C atoms. This gives stability to the cation.
6. We know that, in aromatic compounds, there is delocalisation of electrons among all the C atoms in the ring. But in fig.13.117 above, we see that delocalisation stops at the C atom which is sp3 hybridized. Recall that, delocalisation is possible only if there is a single unhybridized p orbital. Single unhybridized p orbital is available only if the C atom is sp2 hybridized. So it is clear that, arenium ion does not have aromatic character.
(c) Removal of proton from carbocation intermediate
•
One C atom in the arenium ion is sp3 hybridized because, it has to accommodate four bond. If the H atom can be removed from that C atom, then it will revert back to sp2 hybridization. So to restore the aromatic character, the arenium ion releases the H atom. This can take place in two different ways:
1. When the reaction is halogenation, alkylation or acylation:
•
Consider the case where we are adding a Cl atom to the benzene molecule. In this case, [AlCl4]- will be formed in the reaction mixture. (see fig.13.111 of the previous section).
•
Consider the case where we are adding an alkyl group to the benzene molecule. In this case also, [AlCl4]- will be formed in the reaction mixture. (see fig.13.112 of the previous section).
•
Consider the case where we are adding an acyl group to the benzene molecule. In this case also, [AlCl4]- will be formed in the reaction mixture. (see fig.13.113 of the previous section).
•
So in all the above three cases, [AlCl4]- will be present in the reaction mixture.
The carbocation intermediate will react with [AlCl4]-. This is shown in fig.13.118 below:
 |
| Fig.13.118 |
•
First, the [AlCl4]- undergoes heterolytic cleavage to give AlCl3 and Cl-. This is shown in fig.a
•
The two electrons of the C-H bond in the arenium ion, moves to restore the double bond. This is indicated by the curved green arrow in fig.b
•
When the electrons move in this way, the H is removed as a proton. We get the substituted benzene ring.
•
The proton combines with the Cl- to give one molecule of HCl. This is shown in fig.c
2. When the reaction is nitration:
•
Consider the case where we are adding a NO2 group to the benzene
molecule. In this case, [HSO4] - will be formed in the reaction
mixture. (see fig.13.114 of the previous section).
The carbocation intermediate will react with [HSO4] -. This is shown in fig.13.119 below:
 |
| Fig.13.119 |
• The two electrons of the C-H bond in the arenium ion, moves to restore the double bond. This is indicated by the curved green arrow in fig.a
• When the electrons move in this way, the H is removed as a proton. We get the substituted benzene ring.
• The proton combines with the [HSO4] - to give one molecule of H2SO4. This is shown in fig.b
Now we know how a substitution reaction takes place. Let us see some actual substitution reactions. We have to learn about five reactions. They are: (i) Nitration, (ii) Halogenation (iii) Friedel-Crafts alkylation (iv) Friedel-Crafts acylation (v) Sulphonation
(i) Nitration
•
This can be written in 4 steps:
1. The first step is the formation of the electrophile. In our present case, it is $\rm{N^{\oplus} O_2}$. We saw it's formation in fig.13.114 and fig.13.115 in the previous section.
2. In the second step, this electrophile gets attached to the benzene ring to form a carbocation. We saw it in fig.13.117 in this section.
3. In the third step, a H atom is removed from the carbocation. We saw it in fig.13.119 in this section.
4. So we can write the overall reaction as:
•
Benzene is heated with a mixture of concentrated nitric acid and concentrated sulphuric acid. As a result, a nitro group is introduced into the benzene ring.
•
The equation is shown in fig.13.120 below:
 |
| Fig.13.120 |
•
A mixture of concentrated nitric acid and concentrated sulphuric acid is called nitrating mixture.
(ii) Halogenation
•
This can be written in 4 steps:
1. The first step is the formation of
the electrophile. In our present case, it is $\rm{Cl^{\oplus}}$. We saw
it's formation in fig.13.111 in the previous section.
2.
In the second step, this electrophile gets attached to the benzene ring
to form a carbocation. We saw it in fig.13.117 in this section.
3. In the third step, a H atom is removed from the carbocation. We saw it in fig.13.119 in this section.
4. So we can write the overall reaction as:
• Arenes reacts with halogens in the presence of a Lewis acid like anhydrous FeCl3, FeBr3 or AlCl3 to yield haloarenes.
•
The equation is shown in fig.13.121 below:
 |
| Fig.13.121 |
5. If excess halogen molecules are used, more H atoms will be replaced from the benzene ring.
• For example, if we use excess chlorine molecules, all H atoms in the benzene ring will be replaced by Cl atoms. This will result in the formation of hexachlorobenzene (C6Cl6). The equation is shown in fig.13.122 below:
 |
| Fig.13.122 |
(iii) Friedel-Crafts alkylation
•
This can be written in 5 steps:
1. The first step is the formation of
the electrophile. In our present case, it is $\rm{C^{\oplus} H_3}$. We saw
it's formation in fig.13.112 in the previous section.
2.
In the second step, this electrophile gets attached to the benzene ring
to form a carbocation. We saw it in fig.13.117 in this section.
3. In the third step, a H atom is removed from the carbocation. We saw it in fig.13.119 in this section.
4. So we can write the overall reaction as:
• When benzene is treated with an alkyl halide in the presence of a Lewis acid like anhydrous AlCl3, alkyl benzene is formed.
•
Two examples are shown in fig.13.123 below:
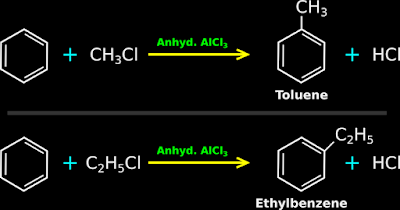 |
| Fig.13.123 |
5. Let us see an interesting case related to alkylation. It can be written in 4 steps:
(i) In the above examples:
♦ When chloromethane was taken, we obtained methylbenzene (toluene).
♦ When chloroethane was taken, we obtained ethylbenzene.
(ii) So if we take 1-chloropropane, we would expect n-propyl benzene.
♦ n-propyl benzene is shown in fig.13.124(a) below.
(iii) But the actual product is isopropyl benzene.
♦ isopropyl benzene is shown in fig.13.124(b) below.
 |
| Fig.13.124 |
(iv) The reason is:
• 1-chloropropane will undergo heterolytic cleavage to give $\rm{CH_3 - CH_2 -C^{\oplus} H_2}$
• In this electrophile, the +ve charge is carried by a primary C atom. There fore it has less stability.
• To achieve stability, it undergoes rearrangement to a form in which the +ve charge is carried by a secondary C atom: $\rm{CH_3 - C^{\oplus}H -C H_3}$
• So in the reaction mixture, the quantity of $\rm{CH_3 - C^{\oplus}H -C H_3}$ will be greater.
• As a result, we will get isopropyl benzene as the major product.
(iv) Friedel-Crafts acylation
•
This can be written in steps:
1. The first step is the formation of
the electrophile. In our present case, it is $\rm{CH_3 C^{\oplus} O}$. We saw
it's formation in fig.13.113 in the previous section.
2.
In the second step, this electrophile gets attached to the benzene ring
to form a carbocation. We saw it in fig.13.117 in this section.
3. In the third step, a H atom is removed from the carbocation. We saw it in fig.13.119 in this section.
4. So we can write the overall reaction as:
• When benzene is treated with an alcyl halide or acid anhydride in the presence of a Lewis acid like anhydrous AlCl3, alcyl benzene is formed.
• Two examples are shown in fig.13.125 below:
 |
| Fig.13.125 |
(v) Sulphonation
•
This can be written in 3 steps:
1. In the case of sulphonation, the first step is not required. This is because, sulphur trioxide (SO3) which is one of the reactants, is already an electrophile.
• The reason for SO3 becoming an electrophile, can be written in 3 steps:
(i) The O atoms being highly electronegative, pulls the electrons from the S atom.
(ii) The S atom thus gains a partial +ve charge.
(iii) This S atom can gain electrons from electron donors.
2.
The remaining steps are the same:
• The electrophile gets attached to the benzene ring
to form a carbocation. We saw it in fig.13.117 in this section.
• Finally, a H atom is removed from the carbocation. We saw it in fig.13.119 in this section.
3. So we can write the overall reaction as:
•
When benzene is heated with fuming sulphuric acid, benzene sulphonic acid is formed.
•
The equation is shown in fig.13.126 below:
 |
| Fig.13.126 |
We have completed a discussion on electrophilic substitution reactions of aromatic compounds. In the next section, we will see addition reactions.
Copyright©2023 Higher secondary chemistry.blogspot.com
No comments:
Post a Comment