In the previous section, we completed a discussion on the chemical properties of alkanes. In this section, we will see conformations.
Some basics can be written in 6 steps:
1. Consider the molecule of ethane.
• We know that, the ethane molecule is made up of two CH3 groups.
• Also we have seen that, the two groups can rotate abour the C-C sigma bond. [Animation in fig.4.141 of section 4.25].
2. Due to such rotation, the H atoms will occupy different positions in space.
3. When the H atoms are at maximum possible distances away from each other, the molecule as a whole will have a low amount of energy.
• This is because, at greater distances, the repulsion between electrons will be lesser.
4. When the H atoms are at closest possible distances from each other, the molecule as a whole, will have a higher amount of energy.
• This is because, at lesser distances, the repulsion between electrons will be greater.
5. So it is important to learn about the various possible positions of the H atoms.
6. The various possible arrangements of atoms in space are known as conformations.
First we will see eclipsed conformation. It can be explained in 8 steps:
1. Fig.13.41 below shows the ball and stick model of an ethane molecule.
♦ The two C atoms are named as C1 and C2.
♦ The six H atoms are named as H1, H2, H3, . . . H6.
 |
| Fig.13.41 |
2. Assume that:
• C1 and it's three H atoms (H1, H2 and H3) are stationary
• C2 and it’s three H atoms (H4, H5 and H6) are rotating about the C1-C2 axis.
3. Now consider fig.13.42(a) below:
 |
| Fig.13.42 |
• At a certain instant during the rotation, a line (shown in yellow color) is drawn through H1.
• This line is parallel to the C1-C2 axis.
• This line passes through H4.
4. In this position, H4 is aligned with H1.
♦ Also H2 is aligned with H5.
♦ Also H3 is aligned with H6.
5. In this position, if a person look at the molecule from the tail end of the red arrow (red arrow is the C1-C2 axis), H4 will not be visible.
• This is because, H1 will be obstructing the view.
• Similarly, H2 will be obstructing the view of H5.
• Similarly, H3 will be obstructing the view of H6.
◼ The person will be able to see only three H atoms: H1, H2 and H3.
◼ In this situation, the conformation is called eclipsed conformation.
6. It is not convenient to draw the ball and stick model every time. So we use two simplified methods to represent various conformations:
♦ Sawhorse projections
♦ Newman’s projections.
7. Sawhorse projections can be explained in 3 steps:
(i) The C1-C2 bond is shown as a longer line.
♦ This line is drawn in a tilted manner.
♦ That is., upper end of the line is towards the right with respect to the lower end.
♦ This is the pink line in fig.13.42(b) above.
♦ The front C atom is assumed to be at the lower end of the line.
♦ The rear C atom is assumed to be at the upper end of the line.
(ii) Each C atom will have three lines attached to them.
• Those three lines are shown in brown color in fig.13.42(b).
• The angle between these brown lines is 120o.
• The three lines represent the three H atoms.
♦ Let us recall how 120o is obtained.
♦ In the tetrahedral arrangement, the three H atoms are situated at the three corners of an equilateral triangle. This is shown in fig.13.43 below:
 |
| 13.43 |
♦ The projected position of the C atom will be at the centroid of the equilateral triangle.
♦ The 120o angle is the central angle.
(iii) The Sawhorse projection gives us a clear picture about the arrangements of H atoms in space.
8. Next we will see Newman’s projection. It can be explained in 5 steps
(i) It is assumed that, the person who views the molecule, looks at the C1-C2 bond head on.
(ii) The front C atom is represented as a dot. The brown dot in fig.13.42(c)
• The rear C atom is represented as a circle. The red circle in fig.(c).
(iii) Three lines are attached to the front C atom (the brown dot). Those three lines are shown in brown color in fig.c. The angle between those brown lines is 120o. The three lines represent the three H atoms.
(iv) Three lines are attached to the rear C atom (the red circle). Those three lines are shown in red color in fig.c.
• The angle between those red lines is 120o.
• The three lines represent the three H atoms.
(v) Theoretically, the three red lines must be exactly behind the three brown lines.
• But then the red lines will become invisible. To convey the information that the three H atoms of the rear C atom are actually present, we draw them slightly out of line.
So now we have a basic idea about eclipsed conformation. Next we will see the staggered conformation. it can be explained in 4 steps.
1. In fig.13.42 above, the H atoms in the two CH3 groups are aligned. But as the rotation continues, the alignment will be lost.
• Let us assume that, the aligned position in fig.13.42 is the initial position. From this position, if a rotation of 60o take place, the H atoms will be at the positions shown in fig.13.44(a) below. Note that, the yellow line through H1 no longer passes through H4.
 |
| Fig.13.44 |
2. Fig.a shows the arrangement in which:
♦ the C-H bonds of the C2 atom
♦ are at the maximum possible distance
♦ from the C-H bonds of the C1 atom.
3. If we increase the angle of rotation above 60o, will the distance increase?
• The answer is 'no'. In fact, if we increase the angle, the distance will decrease.
• This is because, when the angle is increased,
♦ H4 will become closer to H2.
♦ H5 will become closer to H3.
♦ H6 will become closer to H1.
4. So it is clear that, for maximum separation, the angle must be 60o.
◼ When the angle is 60o, the conformation is called staggered conformation.
♦ Fig.13.44(b) shows the staggered conformation using Sawhorse projection.
♦ Fig.13.44(c) shows the staggered conformation using Newman’s projection.
So we have seen eclipsed conformation and staggered conformation. Next we will see skew conformation. It can be written in 3 steps:
1. Eclipsed conformation is obtained when the H atoms in one CH3 group is aligned with the H atoms in the other CH3 group.
• Staggered conformation is obtained when the angle of rotation is 60o from the aligned position.
2. But angle of rotation can be any angle between 0o and 360o.
• So infinite number of angles are possible.
• Correspondingly, we get infinite number of spatial arrangements.
3. Out of the infinite possible arrangements, one will be the eclipsed conformation and another will be staggered conformation.
• Consider the arrangements other than eclipsed and staggered. Each of those arrangements is called a skew conformation.
Now we have a detailed understanding about various conformations. We will write the definition of conformations. It can be written in 5 steps:
1. In alkanes, free rotation about the C-C single bond is possible.
2. Due to this rotation, we get different arrangements of atoms in space.
◼ These different spatial arrangements are called conformations.
3. The various conformations can change into one another.
4. Another name for conformations is conformers
• Yet another name for conformations is rotomers.
5. In all the conformations, the bond angles and bond lengths will be the same.
Relative stability of conformations
A comparison between the stability of various conformations can be written in 6 steps:
1. In the staggered conformation, we have seen that:
♦ C-H bonds of the rear C atom
♦ are at maximum possible distance from the
♦ C-H bonds in the front C atom.
• So in the staggered conformation,
♦ the repulsion between
♦ electron clouds of the front C-H bonds and
♦ electron clouds of the rear C-H bonds
♦ will be minimum.
• Minimum repulsion will lead to minimum energy.
• Minimum energy gives maximum stability to the molecule as a whole.
2. In the eclipsed conformation, we have seen that:
♦ C-H bonds of the rear C atom
♦ are at minimum possible distance from the
♦ C-H bonds in the front C atom.
• So in the eclipsed conformation,
♦ the repulsion between
♦ electron clouds of the front C-H bonds and
♦ electron clouds of the rear C-H bonds
♦ will be maximum.
• To counter the increased repulsion, the molecule will need to possess greater energy.
• Due to the greater energy, the molecule as a whole, will be less stable.
3. So two points are clear:
(i) In the staggered conformation, the molecule possess the minimum possible energy and maximum possible stability.
(ii) In the eclipsed conformation, the molecule possess the maximum possible energy and minimum possible stability.
4. So the molecule in the staggered conformation will not want to change into eclipsed conformation.
• We will need to supply energy for the change to take place.
5. Suppose that, initially, the molecule is in the staggered conformation. This is shown in fig.13.45(a) below:
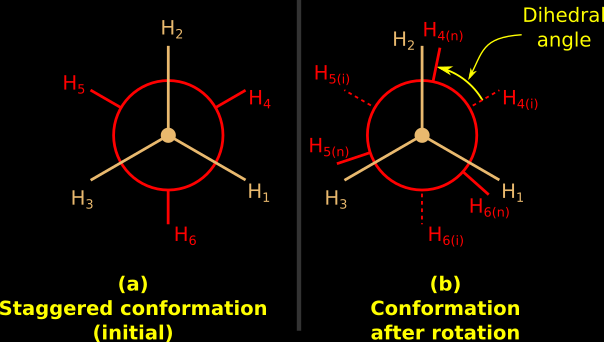 |
| Fig.13.45 |
• If we give some energy, it will begin to rotate. The angle of rotation will depend upon the quantity of energy supplied.
• In other words, the amount of energy supplied can be related to the angle of rotation.
• So if we measure the angle of rotation, we will be able to find the amount of energy supplied.
• This angle of rotation starting from the staggered conformation is known as angle of torsion.
• Another name for angle of torsion is dihedral angle.
• The quantity of energy supplied to achieve the angle of torsion is known as torsional strain.
• In fig.13.45(b), the initial position of H4 is H4(i). After rotation, it's new position is H4(n). So the dihedral angle can be clearly marked.
6. In step (4), we saw that:
• Energy needs to be supplied to change from staggered conformation to eclipsed conformation.
• But the energy difference between the two conformations is small. Only about 12.5 kJ per mol.
• Since this is a small quantity of energy, all molecules at room temperature, will possess the required energy.
• So at room temperature, all molecules of ethane will be rotating.
• Rotations will be stopped only if the surroundings are very cold.
We have completed a discussion on alkanes. In the next section we will see alkenes.
Copyright©2022 Higher secondary chemistry.blogspot.com
No comments:
Post a Comment