In the previous section, we saw the details about resonance effect. In this section, we will see electromeric effect.
• The resonance effect that we saw in the previous section, is a permanent effect. For each case that we saw, there were various resonance structures. The molecule exists as the hybrid of those resonance structures.
• But electromeric effect is a temporary effect. This effect occurs in a substrate only when an attacking reagent comes to the vicinity of that substrate. The effect disappears if the attacking reagent is removed from the vicinity of the substrate.
This can be demonstrated using two examples.
Example 1:
This can be written in 4 steps:
1. The fig.12.103 below shows the reaction between ethene and H+ ion. All atoms of ethene have octet.
 |
| Fig.12.103 |
2. When the H+ ion approaches the ethene molecule, the two electrons in the 𝜋 bond moves to the right side C atom.
• The right side C atom uses those two electrons to form a bond with H+
• The left side C atom acquires a +ve charge.
3. Let us write the three salient features of this reaction:
(i) Transfer of electrons occurred due to the presence of the attacking reagent H+.
(ii) Electrons in the 𝜋 bond are transferred.
(iii) After the transfer, the electrons were acquired by one of the atoms present on either ends of the 𝜋 bond.
• The reagent H+ got attached to the atom which acquired the electrons.
(iv) Effect is temporary:
• The electron transfer indicated by the curved-arrow takes place when the H+ come into the vicinity of the ethene molecule.
• If the H+ is taken away from the vicinity of the ethene molecule, the electrons move back to their original positions.
4. If the four conditions mentioned in the above step are satisfied, it is known as positive electromeric effect (+E effect)
Example 2:
This can be written in 4 steps:
1. The fig.12.104 below shows the reaction between ethene and CN- ion. All atoms of ethene have octet.
 |
| Fig.12.104 |
2. When the CN- ion approaches the ethene molecule, the two electrons in the 𝜋 bond moves to the right side C atom.
• The left side C atom thus loses an electron.
• The incoming CN- ion supplies the required electron and forms a bond with the left side C atom.
• The right side C atom acquires a - ve charge.
3. Let us write the three salient features of this reaction:
(i) Transfer of electrons occurred due to the presence of the attacking reagent CN-.
(ii) Electrons in the 𝜋 bond are transferred.
(iii) After the transfer, the electrons were acquired by one of the atoms present on either ends of the original 𝜋 bond.
• The reagent CN- got attached to the other atom which did not acquire electrons.
(iv) Effect is temporary:
• The electron transfer indicated by the curved-arrow takes place when the CN- come into the vicinity of the ethene molecule.
• If the CN- is taken away from the vicinity of the ethene molecule, the electrons move back to their original positions.
4. If the four conditions mentioned in the above step are satisfied, it is known as negative electromeric effect (-E effect)
◼ What happens when inductive effect and electromeric effect act at the same time?
• The answer can be written in steps:
1. We have seen inductive effect in an earlier section. Inductive effect also involves pulling of electrons.
2. It may so happen that:
• Due to the inductive effect, electrons tend to move in a particular direction.
• But due to the electromeric effect, the electrons tend to move in the opposite direction.
3. Then there will be a competition between the two effects.
• If such a situation occur, electromeric effect will be the winner.
Hyperconjugation
• Some basics of hyperconjugation can be written based on ethyl cation. It can be written in 9 steps:
1. We know the structure of ethane: CH3ㅡCH3. The two C atoms, carry three H atoms each.
• If one H atom leaves (taking both electrons in the bond with it), we get the ethyl cation. We write it as: $\mathbf{\rm{CH_3\overset{+}{C}H_2}}$
• We saw it’s structure when we discussed heterolytic cleavage. See fig.12.69(a) of section 12.10.
2. So the ethyl cation has two parts:
♦ $\mathbf{\rm{CH_3}}$ㅡ
♦ ㅡ$\mathbf{\rm{\overset{+}{C}H_2}}$
• The C atom in CH3 part has octet. That C atom is sp3 hybridized. It is shown in fig.12.105(a) below. The H atoms in this part are named as HA, HB and HC
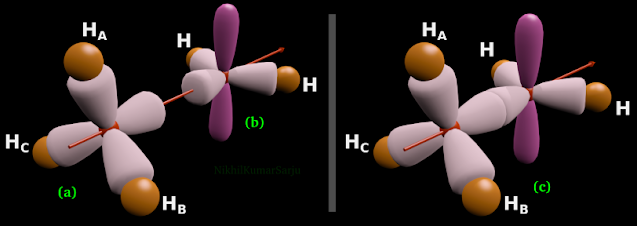 |
| Fig.12.105 |
• The C atom in CH2 part has only sextet. That C atom is sp2 hybridized. It is shown in fig.12.105(b) above. We already know how this structure is derived. See fig.12.71 of section 12.10.
3. Figs (a) and (b) show us the two parts separately. When they combine together, we get the ethyl cation. It is shown in fig.c
• They are combined together through a σ bond between the two C atoms.
4. Though the two parts are combined together, rotation can take place about the red axis. We saw this in the animation in fig.4.141 of section 4.25.
• Consider a particular instant during the rotation. At that instant, the HA atom and the hybrid orbital carrying HA, comes in alignment (same plane) with the empty p-orbital of the CH2 part.
• In such a situation, the two electrons in the CㅡHA bond will get delocalized into the empty p-orbital.
• This is shown in fig.12.106(a) below. This type of delocalization is known as hyperconjugation.
 |
| Fig.12.106 |
5. As a result, HA will lose both electrons in the bond.
• Those two electrons will be used to form a 𝜋 bond between the two C atoms.
♦ It is a 𝜋 bond because, the overlap is lateral.
• Thus the original CㅡC single bond becomes a double bond C=C.
• The structural formula of the resulting structure will be as shown in fig.12.106(b) above. In that fig., we see that, HA has no bond with C. Also, HA has a + charge. This is due to the loss of one electron. The C has gained an electron. So it's original + charge disappears.
6. Now, as the rotation continues, at another instant, the HB atom and the hybrid orbital carrying HB, comes in alignment (same plane) with the empty p-orbital of the CH2 part.
• At that instant, the two electrons in the CㅡHB bond will get delocalized into the empty p-orbital.
• The structural formula of the resulting structure will be as shown in fig.12.107(a) below. In that fig., we see that, HB has no bond with C. Also, HB has a + charge. This is due to the loss of one electron.
 |
| Fig.12.107 |
7. Similarly, as the rotation continues, at another instant, the HC atom and the hybrid orbital carrying HC, comes in alignment (same plane) with the empty p-orbital of the CH2 part.
• At that instant, the two electrons in the CㅡHC bond will get delocalized into the empty p-orbital.
• The structural formula of the resulting structure will be as shown
in fig.12.107(b) above. In that fig., we see that, HC has no bond with
C. Also, HC has a + charge. This is due to the loss of one electron.
8. The structural formulas in figs.12.106 and 107 are all resonance structures of the same ethyl cation.
• The analysis of the resonance structures can be written in 3 steps:
(i) The structure I in fig.12.108 below shows the original structure.
• The red curved arrow indicates that, the two electrons in the CㅡHA bond is transferred to the CㅡC single bond. That single bond thus becomes a double bond. This double bond is shown in II.
• Due to the action of the red curved arrow, the bond between C and HA is lost. There is 'no bond' between C and HA. This is also shown in II.
• The red curved arrow in I, comes into play when the HA atom becomes aligned with the empty p orbital. It is a hyperconjugation.
 |
| Fig.12.108 |
(ii) From (i) above, we know how the structure II is obtained. So now we can analyze the curved-arrows in II.
• The red curved-arrow in II indicates that the CㅡHA receives the two electrons back. So in III, we see the CㅡHA bond.
• But the C=C double bond does not become a single bond. This is because, the HB now becomes aligned with the empty p-orbital and hyperconjugation takes place.
• The transfer of electrons by this hyperconjugation is indicated by the magenta curved-arrow in II. As a result, there is ‘no bond’ between C and HB. We see this in III
(iii) From (ii) above, we know how the structure III is obtained. So now we can analyze the curved arrows in III.
• The red curved-arrow in III indicates that the CㅡHB receives the two electrons back. So in IV, we see the CㅡHB bond.
•
But the C=C double bond does not become a single bond. This is because,
the HC now becomes aligned with the empty p-orbital and
hyperconjugation takes place.
• The transfer of electrons by this
hyperconjugation is indicated by the magenta curved-arrow in III. As a
result, there is ‘no bond’ between C and HC. We see this in IV
9. Now we can write about the stability of the ethyl cation. It can be written in five steps:
(i) Originally, the ethyl cation does not have any double bond. The + charge is carried by one of the C atoms. This is one of the four resonance structures.
(ii) Due to hyperconjugation, the single bond between the two C atoms become a double bond. The + charge is now carried by HA. This is another resonance structure.
(iii) Again due to hyperconjugation, the single bond between the two C atoms become a double bond. The + charge is now carried by HB. This is another resonance structure.
(iv) Again due to hyperconjugation, the single bond between the two C atoms
become a double bond. The + charge is now carried by HC. This is yet another resonance structure.
(v) So in the hybrid structure, the + charge is distributed among four atoms:
One C atom and three H atoms.
• Such a distribution of charge, gives greater stability to the ethyl cation.
In an earlier section, we wrote about the stability of ethyl cation. (step 9 below fig.12.70 of section 12.10). The above nine steps helps us to explain it's stability. In the next section we will see the stability of isopropyl cation.
Copyright©2022 Higher secondary chemistry.blogspot.com
No comments:
Post a Comment