In the previous section, we completed a discussion on thermodynamics. In this section, we will see equilibrium
• We know that, when a chemical reaction takes place, reactants get converted into products
• When the reaction is complete, three types of situation can arise:
Case 1:
• All reactants are converted into products
♦ There may be traces of reactants left
♦ But it may not be possible even to detect those remaining reactants
• We say that:
♦ Quantity of reactants
♦ is far less than
♦ Quantity of products
Case 2:
• Quantity of products formed is very low
♦ Most of the reactants remain as such
• We say that:
♦ Quantity of reactants
♦ is far greater than
♦ Quantity of products
Case 3:
• Reaction stops in the midway
♦ Some products are formed
♦ Some reactants remain as such
• We say that:
♦ Quantity of reactants remaining
♦ is comparable to
♦ Quantity of products newly formed
• Consider case 3
• We encounter this case on many occasions in chemical industries and also in labs
• During the chemical reaction, a point is reached at which, we see no activity
♦ But at that point, all reactants are not converted into products
♦ We call this point: equilibrium
• Let us see the main features of equilibrium. It can be written in 3 steps:
1. At equilibrium, we are inclined to think that, the reaction has stopped. This is because, we see no activity
• But in reality, the reaction is still going on
♦ Only that, the reaction is going on in two opposite directions
• We can say:
♦ In the forward direction, the reactants are being converted into products
♦ In the backward direction, the products are being converted into reactants
2. Then why do we see 'no activity'?
The reason can be written in 3 steps:
(i) There is no change in the quantity of reactants because,
♦ The reactants used up in the forward reaction
♦ is replenished by
♦ The reactants formed from the backward reaction
(ii) There is no change in the quantity of products because,
♦ The products used up in the backward reaction
♦ is replenished by
♦ The products formed from the forward reaction
(iii) Based on (i) and (ii), we can write:
• At equilibrium,
♦ Rate of forward reaction
♦ is equal to
♦ Rate of backward reaction
3. Can we change the equilibrium?
• On many occasions, we may not be satisfied with the quantities obtained at equilibrium
• We may want a greater quantities of products
• This can be achieved by increasing the rate of the forward reaction
• If the rate of forward reaction is increased,
♦ The quantities of products obtained from the forward reaction
♦ in a given time duration
♦ Will be greater than
♦ The quantities of reactants formed from the backward reaction
• A reaction can be forced to proceed in a desired direction by two methods:
(i) Making appropriate changes to the temperature, pressure etc.,
(ii) Making appropriate changes to the concentrations of reactants/products
Solid-Liquid Equilibrium
• Equilibrium occur not only in chemical reactions. It is observed in many physical processes also. Let us see some examples:
• First we will see the equilibrium between solid phase and liquid phase. It can be explained by taking ice and water as an example. It can be written in 8 steps:
1. Consider a mixture of ice and water
♦ Let the temperature of the mixture be 273 K
2. The mixture is placed inside a thermos flask
♦ So no heat enters or leaves the mixture
3. Using precision instruments, we can measure the individual masses of ice and water in the thermos flask
♦ Let the masses of water and ice be mwater and mice respectively
4. After a time duration of say 10 minutes, if we measure the masses, we will see that, mwater and mice are unchanged
• We can say that, water and ice are in a state of equilibrium
5. We observe no activity
• But in reality, activities are taking place at the interface between water and ice
♦ Some water molecules collide with the ice and adhere to it
✰ This can be represented as: H2O(l) → H2O(s)
♦ Some ice molecules escape from the surface of the ice and goes into the liquid
✰ This can be represented as: H2O(s) → H2O(l)
♦ Both the processes are taking place simultaneously
✰ So we can combine them as: H2O(l) ⇌ H2O(s)
6. We can compare equilibrium and non-equilibrium between ice and water:
Case 1:
• If the pressure is 1 atm and temperature is less than 273 K:
♦ The number of water molecules adhering to the ice
♦ will be greater than
♦ The number of ice molecules escaping into water
• Then the quantity of ice will go on increasing
• Also the quantity of water will go on decreasing
• We can write:
♦ In the process: H2O(l) ⇌ H2O(s),
♦ Both the processes takes place simultaneously
♦ But the forward process takes place at a higher rate
♦ This is not an equilibrium
Case 2:
• If the pressure is 1 atm and temperature is greater than 273 K:
♦ The number of water molecules adhering to the ice
♦ will be lesser than
♦ The number of ice molecules escaping into water
• Then the quantity of ice will go on decreasing
• Also the quantity of water will go on increasing
• We can write:
♦ In the process: H2O(l) ⇌ H2O(s),
♦ Both the processes takes place simultaneously
♦ But the backward process takes place at a higher rate
♦ This is not an equilibrium
Case 3:
• If the pressure is 1 atm and temperature is equal to 273 K:
♦ The number of water molecules adhering to the ice
♦ will be equal to
♦ The number of ice molecules escaping into water
• Then the quantity of ice will remain the same
• The quantity of water will also remain the same
• We can write:
♦ In the process: H2O(l) ⇌ H2O(s),
♦ Both the processes takes place simultaneously
♦ Both the processes takes place at the same rate
♦ This is an equilibrium
7. Case 3 is equilibrium
• At equilibrium,
♦ The forward process and the reverse process occur simultaneously
♦ The forward process and the reverse process occur at the same rate
8. The above steps gives us a perfect explanation for equilibrium between liquid phase and solid phase. In fact, the equilibrium is used to define melting point/ freezing point:
| For any pure substance at atmospheric pressure, the temperature at which the solid and liquid phases are at equilibrium is called normal melting point or normal freezing point of the substance |
Liquid-Vapor Equilibrium
• Next we will consider the equilibrium between liquid phase and vapour phase. It can be explained by taking water and water-vapor as an example. It can be written in 8 steps
1. Fig.7.1(a) below shows a transparent box
• It is attached with a U-tube containing mercury
♦ An U-tube containing mercury is called manometer
♦ It is used to determine pressure
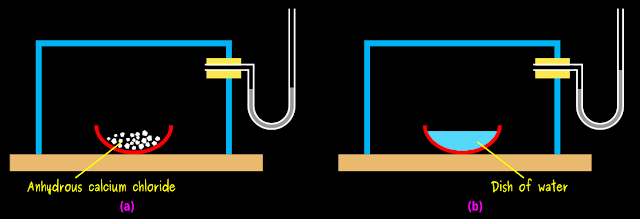 |
| Fig.7.1 |
2. Place a drying agent like anhydrous calcium chloride or (phosphorus penta-oxide) in the box
• The drying agent will absorb all the gaseous water molecules inside the box
• After a few hours,
♦ There will not be any water molecules inside the box
♦ Note the level of mercury in the right limb of the manometer
♦ Remove the drying agent by tilting the box on one side
♦ Quickly place a petri dish containing water inside the box
3. We can observe that:
• The level of mercury in the right limb of the manometer slowly increases
♦ This shows that, the pressure inside the box is slowly increasing
• The volume of water in the petri dish decreases
4. Remember that, no gaseous molecules can enter the box because, the mercury acts as a seal
• So the increase in pressure inside the box must be due to the formation of new gaseous molecules
5. How are the new gaseous molecules formed?
• New gaseous molecules are formed due to the evaporation of water in the dish
• Some of the liquid water molecules become gaseous water molecules
• The decrease in volume of water in the dish is clear evidence for this transformation
6. Due to the formation of new gaseous molecules, the pressure inside the box increases
• This excess pressure pushes the mercury
• Thus the level of mercury in the right limb rises
• But this 'increase in pressure' does not continue indefinitely. After some time, the mercury level becomes static
7. Let us analyze the processes taking place inside the box from the moment when the dish of water is placed. It can be written in 6 steps:
(i) When the water is placed inside the box, some liquid water molecules from the surface of the water, escape from the mass of water
• Once those molecules escape, they are in the gaseous state
• This process can be represented using symbols as:
H2O(l) → H2O(vap)
(ii) This process continues and so, the quantity of gas inside the box increases
• At the same time, the volume of water decreases
(iii) The increase in quantity of gas causes increase in pressure
• This excess pressure pushes the mercury upwards
• That is why we see the rise in mercury level
(iv) All the while when this process is taking place, another process is also taking place simultaneously
• It is the condensation of some of the gaseous water molecules back into the liquid state
• This process can be represented using symbols as:
H2O(vap) → H2O(l)
(v) Since the two processes are taking place simultaneously, we can write:
H2O(l) ⇌ H2O(vap)
• Initially, rate of the forward reaction is greater than rate of backward reaction
• That is.,
♦ Number of molecules entering the gaseous phase
♦ is greater than
♦ Number of molecules leaving the gaseous phase
• So quantity of gas increases, causing the increase in pressure
• The increase in pressure causes the mercury level to rise up
(vi) But after some time, an equilibrium will be reached
• That is.,
♦ Number of molecules entering the gaseous phase
♦ become equal to
♦ Number of molecules leaving the gaseous phase
• So quantity of gas becomes steady, causing no further increase in pressure
• The steady pressure causes the mercury level to remain static
8. So now we know the reason for the two observations:
♦ Mercury level rising initially
♦ Mercury level becoming static after some time
• Next, slightly increase the temperature of the water
• Now more water molecules will have the required energy to break away from the water mass
• So the number of gaseous water molecules inside the box will increase
• This increases the pressure
• The mercury level in the right limb goes up
• If the new temperature is kept constant, a new equilibrium will be reached
Let us see the practical application of the above experiment. It can be written in 7 steps
1. In the above experiment, the water is inside a closed container
• So water molecules cannot escape
2. If we place water in a dish which is open to the atmosphere, the water molecules escaping from the water mass will be blown away by air currents
• The water level in the dish gradually decreases until no water is left
◼ We call this process as: evaporation
• Depending on the surrounding temperature, this process may take several hours to a few days
3. Can we obtain equilibrium when water is open to atmosphere?
• Answer can be written in 4 steps:
(i) When the water is open to atmosphere, we can consider the atmospheric pressure as an invisible lid
• This lid will try to suppress the gaseous water from escaping
(ii) But the air currents blow away the few gaseous water molecules
(iii) This decreases the pressure of the vapour
• Thus the equilibrium between liquid and gaseous molecules is disrupted
♦ This favors the forward process in H2O(l) ⇌ H2O(vap)
• More liquid molecules are able to go into gaseous phase
(iv) Those newly formed molecules are also blown away
• This process continues until no water remains in the dish
◼ So we can write:
Equilibrium between gaseous and liquid molecules cannot be established when water is open to atmosphere
4. Suppose that, there are no air currents. Then there will be equilibrium
• We want to know how such an equilibrium can be disrupted
• It can be written in 8 steps
(i) If there are no air currents, the water level will remain static due to the presence of the invisible lid
(ii) In such a situation, if we increase the temperature of the water, more and more liquid molecules will get sufficient energy to break away from the liquid mass
(iii) At the higher temperature, a new equilibrium will be established
• That is.,
♦ Number of molecules leaving the liquid mass increases to a new value
♦ The number of gaseous molecules condensing back to the liquid phase also increases to that new value
(iv) Note that, at this stage, due to the presence of a greater number of gaseous molecules, the vapour pressure would have increased
(v) If we go on increasing the temperature, the vapour pressure will become so high that, it can push away the invisible lid
• That means, at high temperature, the atmospheric pressure will no longer be able to suppress the vapour pressure
(vi) While increasing the temperature, there will come a point at which, the vapour pressure becomes equal to the atmospheric pressure
• At that point, the water will begin to boil
(vii) If the atmospheric pressure is 1 atm, this boiling of water usually begins at 100 ०C
(viii) Upto 100 ०C, the vapour will be in equilibrium with the liquid. This is because,
♦ No gaseous molecules are able to escape
♦ They are suppressed by the atmospheric pressure
• So upto 100 ०C,
♦ Number of molecules leaving the liquid
♦ will be equal to
♦ Number of molecules condensing back
5. So based on the equilibrium between vapour and liquid, we now have a method to define boiling point:
| For any pure liquid at one atmospheric pressure (1.013 bar), the temperature at which the liquid and vapors are at equilibrium is called normal boiling point of the liquid. |
• That means, the vapour can push away that low atmospheric pressure even at a lower temperature
• That is why, at high altitudes, water boil at lower temperatures
7. Let us note an important point. It can be written in 3 steps:
(i) Even if the atmospheric pressure is the same, some liquids have lower boiling points than water
(ii) This is because, such liquids have lower inter molecular attractions
♦ When in liquid phase, their molecules can easily break away from each other
♦ So they can leave the liquid mass easily
(iii) That is why they boil at lower temperatures
• In the next section, we will see equilibrium between solid phase and gaseous
Previous
Contents
Next
Copyright©2021 Higher secondary chemistry.blogspot.com
No comments:
Post a Comment