In the previous section, we saw Lewis symbols. In this section, we will see Octet rule. We will also see details about covalent bonding and Lewis dot structures
The octet rule can be written in 5 steps:
1. Atoms combine with each other. This combination can be achieved by two methods:
(i) Ionic bond
• In this method, there is 'transfer of one or more valence electrons' from one atom to the other
♦ One of the atom loses one or more of it’s valence electrons
✰ The other atom accepts those electrons
(ii) Covalent bond
In this method, there is 'sharing of one or more pairs of electrons' between atoms
♦ All the ‘member electrons’ of all the ‘shared pairs’ will belong to both the atoms
✰ If one pair is shared, the two electrons in that pair will belong to both the atoms
✰ If two pairs are shared, the four electrons in those two pairs will belong to both the atoms
✰ So on . . .
2. When the combination of the atoms is completed, ‘each atom in the combination’ will have 8 electrons in their outermost shell
3. When the 8 electrons are attained, we say this: The atom has attained octet
♦ An atom which has attained octet is stable
♦ In other words, the atom which has octet, has a stable electronic configuration
4. Every atom tries to attain stability
• For attaining stability, the atoms need to attain octet
• For attaining octet, the atoms need to combine with other atoms
■ So we can write:
The atoms combine with other atoms in order to attain octet and stability. This is known as octet rule
5. Kossel and Lewis put forward this theory in 1916. It is known as: the electronic theory of chemical bonding
• Now we will see some details about the covalent bond
• The theory about covalent bond was developed in 1919 by the American scientist Irving Langmuir
• The works of Langmuir was based upon the earlier theory put forward by Kossel and Lewis
• The theory of covalent bonding can be easily understood if we take the Cl2 molecule as an example. We will write it in steps:
1. Consider a Cl atom
• The electronic configuration of Cl is [Ne]3s23p5
• It is clear that, Cl requires one more electron to attain octet
2. Consider the situation where there is a second Cl atom nearby
• If this 2nd Cl atom can donate an electron, the 1st Cl can attain octet
• But then, the 2nd Cl atom will become 'more electron deficient'. So it will not donate it's electron
3. In such a situation, the solution is this:
• The 2nd Cl atom allows the 1st Cl atom to share an electron
• That is., the 1st Cl atom is allowed to use one electron of the 2nd Cl atom
4. But the 2nd Cl atom is still in need of one electron
• So the 2nd Cl atom is allowed to use one electron of the 1st Cl atom
5. In short, we can write:
♦ The 1st Cl atom has claim on one electron of the 2nd Cl atom
♦ The 2nd Cl atom has claim on one electron of the 1st Cl atom
• That is., one pair of electrons is shared by two Cl atoms
♦ Remember that, a 'pair' means 'two'
♦ So 'two electrons' are shared
♦ This can be demonstrated using Lewis symbols. It is shown in fig.4.9(a) below:
• All electrons of the first Cl atom are shown in green color
• All electrons of the second Cl atom are shown in red color
6. In the above fig.4.9(a), on the right side of the arrow, we have the 'product'
• This 'product' gives a clear idea about the structure of the 'resulting atom'
• The dots represent electrons. Such structures are referred to as: Lewis dot structures
■ In the Lewis dot structure, we notice the following 5 points:
(i) The first Cl atom is a green region
(ii) The second Cl atom is a red region
(iii) The two regions overlap at a small central portion
♦ We can call this overlapping portion as: the 'shared region'
✰ 'shared region' is the 'region common to two atoms'
(iv) The 'electrons in the shared region' are the 'electrons which are shared'
(v) The 'electrons outside the shared region' do not take part in any sharing
• There is a special name for these 'outside electrons': lone pairs
• This is because, 'number of these electrons' will be always even. So they can be grouped into 'pairs'
• For example:
♦ If there are 2 'outside electrons', we have one lone pair
♦ If there are 4 'outside electrons', we have two lone pairs
♦ If there are 6 'outside electrons', we have three lone pairs
♦ so on . . .
7. Note that, both the Cl atoms have equal claims on both the electrons in the 'shared pair'
• So the two Cl atoms cannot separate away from each other
8. Once the sharing has taken place, we say this:
■ The two Cl atoms are connected together by a single covalent bond
9. Using appropriate symbols:
• When 'one pair of electrons' is shared, it results in a single covalent bond or single bond
♦ We put a '─' between the two atoms
• When 'two pairs of electrons' is shared, it results in a double covalent bond or double bond
♦ We put a '=' between the two atoms
• When 'three pairs of electrons' is shared, it results in a triple covalent bond or triple bond
♦ We put a '☰' between the two atoms
8. So the structure of Cl2 molecule can be represented as Cl─Cl
• While using such 'shortened form' to represent the structure, we must add some additional information
• This can be explained in 2 steps:
(i) We have electrons inside the 'shared region'
♦ All information about these 'shared electrons' can be conveyed using '─' OR '=' OR '☰'
(i) We have electrons outside the 'shared region'. They are the 'lone pairs'
♦ We must covey the information about these 'lone pairs' also
♦ For that, we must put appropriate number of dots around the symbols of the atom
♦ This is shown in fig.4.9(b)
• In the fig.b, we have 6 green dots
♦ They represent the '3 lone pairs' in the green region in fig.a
• In the fig.b, we have 6 red dots
♦ They represent the '3 lone pairs' in the red region in fig.a
• We can draw Lewis dot structures of a large number of compounds
• We will encounter two types:
Type 1: Same atoms are present in the compound
• Examples:
♦ All atoms in O2 are O
♦ All atoms in F2 are F
Type 2: Different atoms are present in the compound
Examples:
♦ H and O atoms are present in H2O
♦ C and Cl atoms are present in CCl4
■ While studying Lewis dot structures, the following 4 points must be kept in mind:
1. Number of bonds:
• When we see a single bond (‘─’), it indicates ‘sharing of two electrons (a pair)’
♦ Conversely, when we see ‘two electrons (a pair) in the shared region’, it indicates a ‘─’
✰ 'shared region' is the 'region common to two atoms'
• When we see a double bond (‘=’), it indicates ‘sharing of four electrons (two pairs)’
♦ Conversely, when we see ‘four electrons (two pairs) in the shared region’, it indicates a ‘=’
• When we see a triple bond (‘☰’), it indicates ‘sharing of six electrons (three pairs)’
♦ Conversely, when we see ‘six electrons (three pairs) in the shared region’, it indicates a ‘☰’
2. Contribution from each atom
• If we see a ‘─’ between two atoms, it is clear that, each of those two atoms have contributed exactly one electron to make that pair
• If we see a ‘=’ between two atoms, it is clear that, each of those two atoms have contributed exactly two electrons to make those two pairs
• If we see a ‘☰’ between two atoms, it is clear that, each of those two atoms have contributed exactly three electrons to make those three pairs
3. Number of electrons possessed by each atom
• When the Lewis dot structure of a molecule is completed, we must do a check. This check can be done in 3 steps:
(i) Take an atom in the Lewis dot structure
(ii) Count the number of dots around that atom
(iii) This number must be 8
Do this check for each atom in the molecule
4. The shortened form:
• All information about the 'shared electrons' can be conveyed using '─' OR '=' OR '☰'
• All information about the 'lone pairs' should be conveyed using dots around the symbols
Example 1:
• Fig.4.10(a) below shows the Lewis dot structure of H2O
• The 1 valence electron of H is shown in green color
• The 6 valence electrons of O are shown in red color
• We can write the following 4 points:
1. Type of bond
• Consider the ‘shared region’ between the first H and the O
♦ There are two dots in this region
♦ So we put a ‘─’ between the first H and O
♦ This is a single bond
• Consider the ‘shared region’ between the second H and the O
♦ There are two dots in this region
♦ So we put a ‘─’ between the second H and O
♦ This is a single bond
■ So the shortened form is H─O─H
This is shown below the Lewis dot structure in fig.4.10(a)
2. Contribution from each atom
• Consider the ‘─’ between the first H and the O
♦ It is clear that:
✰ One electron in the '─' belongs to the H
✰ The other electron in the '─' belongs to the O
• Consider the ‘─’ between the second H and the O
♦ It is clear that:
✰ One electron in the '─' belongs to the H
✰ The other electron in the '─' belongs to the O
3. Checking the number of electrons in the Lewis dot structure:
• The first H has 2 dots around it
• The O has 8 dots around it
• The second H has 2 dots around it
• When the H attains 2 electrons in the 1s shell, we cannot call it an 'octet'
♦ The word 'oct' is related to '8'. For example, an octagon has 8 sides
■ When H attains the required 2 electrons, we say this:
The H has attained duplet
• The two H atoms do not have any 'lone pairs'
• The two 'lone pairs' of O are indicated by four red dots in the shortened form
Example 2:
• Fig.4.10(b) above shows the Lewis dot structure of CCl4
• The 7 valence electrons of Cl are shown in green color
• The 4 valence electrons of C are shown in red color
• We can write the following 4 points:
1. Type of bond
• Consider the ‘shared region’ between the left Cl and the C
♦ There are two dots in this region
♦ So we put a ‘─’ between the left Cl and C
♦ This is a single bond
• Consider the ‘shared region’ between the right Cl and the C
♦ There are two dots in this region
♦ So we put a ‘─’ between the right Cl and the C
♦ This is a single bond
• Consider the ‘shared region’ between the top Cl and the C
♦ There are two dots in this region
♦ So we put a ‘─’ between the top Cl and the C
♦ This is a single bond
• Consider the ‘shared region’ between the bottom Cl and the C
♦ There are two dots in this region
♦ So we put a ‘─’ between the bottom Cl and the C
♦ This is a single bond
■ The shortened form is shown in the fig.4.10(c)
2. Contribution from each atom
• Consider the ‘─’ between the left Cl and the C
♦ It is clear that:
✰ One electron in the '─' belongs to the Cl
✰ The other electron in the '─' belongs to the C
• Consider the ‘─’ between the right Cl and the C
♦ It is clear that:
✰ One electron in the '─' belongs to the Cl
✰ The other electron in the '─' belongs to the C
• Consider the ‘─’ between the top Cl and the C
♦ It is clear that:
✰ One electron in the '─' belongs to the Cl
✰ The other electron in the '─' belongs to the C
• Consider the ‘─’ between the bottom Cl and the C
♦ It is clear that:
✰ One electron in the '─' belongs to the Cl
✰ The other electron in the '─' belongs to the C
3. Checking the number of electrons in the Lewis dot structure:
• The left Cl has 8 dots around it
• The right Cl has 8 dots around it
• The top Cl has 8 dots around it
• The bottom Cl has 8 dots around it
• The C has 8 dots around it
4. The shortened form must show the 'lone pairs' also
• The C atom do not have any lone pairs
• The 'three lone pairs' of each Cl are indicated by six green dots around each Cl in the shortened form
Example 3:
• Fig.4.11(a) below shows the Lewis dot structure of CO2
• The 6 valence electrons of O are shown in green color
• The 4 valence electrons of C are shown in red color
• We can write the following 4 points:
1. Type of bond
• Consider the ‘shared region’ between the first O and the C
♦ There are 4 dots in this region. '4' indicates '2 pairs'
♦ So we put a ‘=’ between the first O and the C
♦ This is a double bond
• Consider the ‘shared region’ between the second O and the C
♦ There are 4 dots in this region. '4' indicates '2 pairs'
♦ So we put a ‘=’ between the second O and the C
♦ This is a double bond
■ So the shortened form is O=C=O
This is shown below the Lewis dot structure in fig.4.11(a)
2. Contribution from each atom
• Consider the ‘=’ between the first O and the C
♦ It is clear that:
✰ Two electrons in the '=' belongs to the O
✰ The remaining two electrons in the '=' belongs to the C
• Consider the ‘=’ between the second O and the C
♦ It is clear that:
✰ Two electrons in the '=' belongs to the O
✰ The remaining two electrons in the '=' belongs to the C
3. Checking the number of electrons in the Lewis dot structure:
• The first O has 8 dots around it
• The C has 8 dots around it
• The second O has 8 dots around it
4. The shortened form must show the 'lone pairs' also
• The C atom do not have any lone pairs
• The 'two lone pairs' of each O are indicated by four green dots around each O in the shortened form
Example 4:
• Fig.4.11(b) above shows the Lewis dot structure of C2H4
• The 1 valence electron of H is shown in green color
• The 4 valence electrons of C are shown in red color
• We can write the following 4 points:
1. Type of bond
• Consider the ‘shared region’ between the top-left H and the first C
♦ There are two dots in this region
♦ So we put a ‘─’ between this H and C
♦ This is a single bond
• Consider the ‘shared region’ between the bottom-left H and the first C
♦ There are two dots in this region
♦ So we put a ‘─’ between this H and C
♦ This is a single bond
• Consider the ‘shared region’ between the top-right H and the second C
♦ There are two dots in this region
♦ So we put a ‘─’ between this H and C
♦ This is a single bond
• Consider the ‘shared region’ between the bottom-right H and the second C
♦ There are two dots in this region
♦ So we put a ‘─’ between this H and C
♦ This is a single bond
• Consider the ‘shared region’ between the two C atoms
♦ There are 4 dots in this region. '4' indicates '2 pairs'
♦ So we put a ‘=’ between the two C atoms
♦ This is a double bond
■ The shortened form is shown in fig.4.11(c)
2. Contribution from each atom
• Consider the ‘─’ between the top-left H and the first C
♦ It is clear that:
✰ One electron in the '─' belongs to the H
✰ The other electron in the '─' belongs to the C
• Consider the ‘─’ between the bottom-left H and the first C
♦ It is clear that:
✰ One electron in the '─' belongs to the H
✰ The other electron in the '─' belongs to the C
• Consider the ‘─’ between the top-right H and the second C
♦ It is clear that:
✰ One electron in the '─' belongs to the H
✰ The other electron in the '─' belongs to the C
• Consider the ‘─’ between the bottom-right H and the second C
♦ It is clear that:
✰ One electron in the '─' belongs to the H
✰ The other electron in the '─' belongs to the C
• Consider the ‘=’ between the two C atoms
♦ It is clear that:
✰ Two electrons in the '=' belongs to the first C
✰ The remaining two electrons in the '=' belongs to the second C
3. Checking the number of electrons in the Lewis dot structure:
• Each of the four H atoms have 2 dots around them
• Each of the C atoms have 8 dots around them
(Remember that, H needs to attain duplet only)
4. The shortened form must show the 'lone pairs' also
• The C atoms do not have any lone pairs
• The H atoms also do not have any lone pairs
Example 5:
• Fig.4.12(a) below shows the Lewis dot structure of N2
• The 5 valence electrons of the first N are shown in red color
• The 5 valence electrons of the second N are shown in green color
• We can write the following 4 points:
1. Type of bond
• Consider the ‘shared region’ between the two N atoms
♦ There are 6 dots in this region. '6' indicates '3 pairs'
♦ So we put a ‘☰’ between the two N s
♦ This is a triple bond
■ So the shortened form is N☰N
This is shown the Lewis dot structure in fig.4.12(a)
2. Contribution from each atom
• Consider the ‘☰’ between the two N atoms
♦ It is clear that:
✰ Three electrons in the '☰' belongs to the first N
✰ The remaining three electrons in the '☰' belongs to the second N
3. Checking the number of electrons in the Lewis dot structure:
• The first N has 8 dots around it
• The second N has 8 dots around it
4. The shortened form must show the 'lone pairs' also
• The 'one lone pair' of each N are indicated by two dots around each N in the shortened form
Example 6:
• Fig.4.12(b) above shows the Lewis dot structure of C2H2
• The 1 valence electron of H is shown in green color
• The 4 valence electrons of C are shown in red color
• The reader may write all the 4 points in his/her own note books as an exercise
The octet rule can be written in 5 steps:
1. Atoms combine with each other. This combination can be achieved by two methods:
(i) Ionic bond
• In this method, there is 'transfer of one or more valence electrons' from one atom to the other
♦ One of the atom loses one or more of it’s valence electrons
✰ The other atom accepts those electrons
(ii) Covalent bond
In this method, there is 'sharing of one or more pairs of electrons' between atoms
♦ All the ‘member electrons’ of all the ‘shared pairs’ will belong to both the atoms
✰ If one pair is shared, the two electrons in that pair will belong to both the atoms
✰ If two pairs are shared, the four electrons in those two pairs will belong to both the atoms
✰ So on . . .
2. When the combination of the atoms is completed, ‘each atom in the combination’ will have 8 electrons in their outermost shell
3. When the 8 electrons are attained, we say this: The atom has attained octet
♦ An atom which has attained octet is stable
♦ In other words, the atom which has octet, has a stable electronic configuration
4. Every atom tries to attain stability
• For attaining stability, the atoms need to attain octet
• For attaining octet, the atoms need to combine with other atoms
■ So we can write:
The atoms combine with other atoms in order to attain octet and stability. This is known as octet rule
5. Kossel and Lewis put forward this theory in 1916. It is known as: the electronic theory of chemical bonding
Covalent bond
• The theory about covalent bond was developed in 1919 by the American scientist Irving Langmuir
• The works of Langmuir was based upon the earlier theory put forward by Kossel and Lewis
• The theory of covalent bonding can be easily understood if we take the Cl2 molecule as an example. We will write it in steps:
1. Consider a Cl atom
• The electronic configuration of Cl is [Ne]3s23p5
• It is clear that, Cl requires one more electron to attain octet
2. Consider the situation where there is a second Cl atom nearby
• If this 2nd Cl atom can donate an electron, the 1st Cl can attain octet
• But then, the 2nd Cl atom will become 'more electron deficient'. So it will not donate it's electron
3. In such a situation, the solution is this:
• The 2nd Cl atom allows the 1st Cl atom to share an electron
• That is., the 1st Cl atom is allowed to use one electron of the 2nd Cl atom
4. But the 2nd Cl atom is still in need of one electron
• So the 2nd Cl atom is allowed to use one electron of the 1st Cl atom
5. In short, we can write:
♦ The 1st Cl atom has claim on one electron of the 2nd Cl atom
♦ The 2nd Cl atom has claim on one electron of the 1st Cl atom
• That is., one pair of electrons is shared by two Cl atoms
♦ Remember that, a 'pair' means 'two'
♦ So 'two electrons' are shared
♦ This can be demonstrated using Lewis symbols. It is shown in fig.4.9(a) below:
 |
| Fig.4.9 |
• All electrons of the second Cl atom are shown in red color
6. In the above fig.4.9(a), on the right side of the arrow, we have the 'product'
• This 'product' gives a clear idea about the structure of the 'resulting atom'
• The dots represent electrons. Such structures are referred to as: Lewis dot structures
■ In the Lewis dot structure, we notice the following 5 points:
(i) The first Cl atom is a green region
(ii) The second Cl atom is a red region
(iii) The two regions overlap at a small central portion
♦ We can call this overlapping portion as: the 'shared region'
✰ 'shared region' is the 'region common to two atoms'
(iv) The 'electrons in the shared region' are the 'electrons which are shared'
(v) The 'electrons outside the shared region' do not take part in any sharing
• There is a special name for these 'outside electrons': lone pairs
• This is because, 'number of these electrons' will be always even. So they can be grouped into 'pairs'
• For example:
♦ If there are 2 'outside electrons', we have one lone pair
♦ If there are 4 'outside electrons', we have two lone pairs
♦ If there are 6 'outside electrons', we have three lone pairs
♦ so on . . .
7. Note that, both the Cl atoms have equal claims on both the electrons in the 'shared pair'
• So the two Cl atoms cannot separate away from each other
8. Once the sharing has taken place, we say this:
■ The two Cl atoms are connected together by a single covalent bond
9. Using appropriate symbols:
• When 'one pair of electrons' is shared, it results in a single covalent bond or single bond
♦ We put a '─' between the two atoms
• When 'two pairs of electrons' is shared, it results in a double covalent bond or double bond
♦ We put a '=' between the two atoms
• When 'three pairs of electrons' is shared, it results in a triple covalent bond or triple bond
♦ We put a '☰' between the two atoms
8. So the structure of Cl2 molecule can be represented as Cl─Cl
• While using such 'shortened form' to represent the structure, we must add some additional information
• This can be explained in 2 steps:
(i) We have electrons inside the 'shared region'
♦ All information about these 'shared electrons' can be conveyed using '─' OR '=' OR '☰'
(i) We have electrons outside the 'shared region'. They are the 'lone pairs'
♦ We must covey the information about these 'lone pairs' also
♦ For that, we must put appropriate number of dots around the symbols of the atom
♦ This is shown in fig.4.9(b)
• In the fig.b, we have 6 green dots
♦ They represent the '3 lone pairs' in the green region in fig.a
• In the fig.b, we have 6 red dots
♦ They represent the '3 lone pairs' in the red region in fig.a
• So we have completed a discussion on the basics of covalent bonds. Next we will see some more details about Lewis dot structures
• We will encounter two types:
Type 1: Same atoms are present in the compound
• Examples:
♦ All atoms in O2 are O
♦ All atoms in F2 are F
Type 2: Different atoms are present in the compound
Examples:
♦ H and O atoms are present in H2O
♦ C and Cl atoms are present in CCl4
■ While studying Lewis dot structures, the following 4 points must be kept in mind:
1. Number of bonds:
• When we see a single bond (‘─’), it indicates ‘sharing of two electrons (a pair)’
♦ Conversely, when we see ‘two electrons (a pair) in the shared region’, it indicates a ‘─’
✰ 'shared region' is the 'region common to two atoms'
• When we see a double bond (‘=’), it indicates ‘sharing of four electrons (two pairs)’
♦ Conversely, when we see ‘four electrons (two pairs) in the shared region’, it indicates a ‘=’
• When we see a triple bond (‘☰’), it indicates ‘sharing of six electrons (three pairs)’
♦ Conversely, when we see ‘six electrons (three pairs) in the shared region’, it indicates a ‘☰’
2. Contribution from each atom
• If we see a ‘─’ between two atoms, it is clear that, each of those two atoms have contributed exactly one electron to make that pair
• If we see a ‘=’ between two atoms, it is clear that, each of those two atoms have contributed exactly two electrons to make those two pairs
• If we see a ‘☰’ between two atoms, it is clear that, each of those two atoms have contributed exactly three electrons to make those three pairs
3. Number of electrons possessed by each atom
• When the Lewis dot structure of a molecule is completed, we must do a check. This check can be done in 3 steps:
(i) Take an atom in the Lewis dot structure
(ii) Count the number of dots around that atom
(iii) This number must be 8
Do this check for each atom in the molecule
4. The shortened form:
• All information about the 'shared electrons' can be conveyed using '─' OR '=' OR '☰'
• All information about the 'lone pairs' should be conveyed using dots around the symbols
Now we will see some examples:
• Fig.4.10(a) below shows the Lewis dot structure of H2O
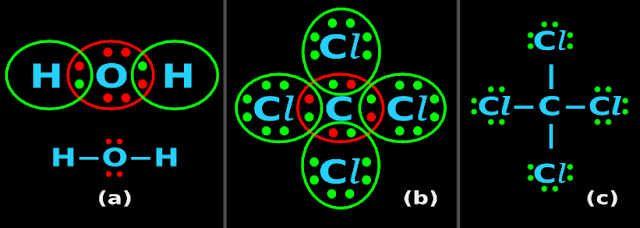 |
| Fig.4.10 |
• The 6 valence electrons of O are shown in red color
• We can write the following 4 points:
1. Type of bond
• Consider the ‘shared region’ between the first H and the O
♦ There are two dots in this region
♦ So we put a ‘─’ between the first H and O
♦ This is a single bond
• Consider the ‘shared region’ between the second H and the O
♦ There are two dots in this region
♦ So we put a ‘─’ between the second H and O
♦ This is a single bond
■ So the shortened form is H─O─H
This is shown below the Lewis dot structure in fig.4.10(a)
2. Contribution from each atom
• Consider the ‘─’ between the first H and the O
♦ It is clear that:
✰ One electron in the '─' belongs to the H
✰ The other electron in the '─' belongs to the O
• Consider the ‘─’ between the second H and the O
♦ It is clear that:
✰ One electron in the '─' belongs to the H
✰ The other electron in the '─' belongs to the O
3. Checking the number of electrons in the Lewis dot structure:
• The first H has 2 dots around it
• The O has 8 dots around it
• The second H has 2 dots around it
• The H atom needs only 2 electrons to fill it's 1s shell
♦ The word 'oct' is related to '8'. For example, an octagon has 8 sides
■ When H attains the required 2 electrons, we say this:
The H has attained duplet
4. The shortened form must show the 'lone pairs' also
• The two 'lone pairs' of O are indicated by four red dots in the shortened form
Example 2:
• Fig.4.10(b) above shows the Lewis dot structure of CCl4
• The 7 valence electrons of Cl are shown in green color
• The 4 valence electrons of C are shown in red color
• We can write the following 4 points:
1. Type of bond
• Consider the ‘shared region’ between the left Cl and the C
♦ There are two dots in this region
♦ So we put a ‘─’ between the left Cl and C
♦ This is a single bond
• Consider the ‘shared region’ between the right Cl and the C
♦ There are two dots in this region
♦ So we put a ‘─’ between the right Cl and the C
♦ This is a single bond
• Consider the ‘shared region’ between the top Cl and the C
♦ There are two dots in this region
♦ So we put a ‘─’ between the top Cl and the C
♦ This is a single bond
• Consider the ‘shared region’ between the bottom Cl and the C
♦ There are two dots in this region
♦ So we put a ‘─’ between the bottom Cl and the C
♦ This is a single bond
■ The shortened form is shown in the fig.4.10(c)
2. Contribution from each atom
• Consider the ‘─’ between the left Cl and the C
♦ It is clear that:
✰ One electron in the '─' belongs to the Cl
✰ The other electron in the '─' belongs to the C
• Consider the ‘─’ between the right Cl and the C
♦ It is clear that:
✰ One electron in the '─' belongs to the Cl
✰ The other electron in the '─' belongs to the C
• Consider the ‘─’ between the top Cl and the C
♦ It is clear that:
✰ One electron in the '─' belongs to the Cl
✰ The other electron in the '─' belongs to the C
• Consider the ‘─’ between the bottom Cl and the C
♦ It is clear that:
✰ One electron in the '─' belongs to the Cl
✰ The other electron in the '─' belongs to the C
3. Checking the number of electrons in the Lewis dot structure:
• The left Cl has 8 dots around it
• The right Cl has 8 dots around it
• The top Cl has 8 dots around it
• The bottom Cl has 8 dots around it
• The C has 8 dots around it
4. The shortened form must show the 'lone pairs' also
• The C atom do not have any lone pairs
• The 'three lone pairs' of each Cl are indicated by six green dots around each Cl in the shortened form
Example 3:
• Fig.4.11(a) below shows the Lewis dot structure of CO2
 |
| Fig.4.11 |
• The 4 valence electrons of C are shown in red color
• We can write the following 4 points:
1. Type of bond
• Consider the ‘shared region’ between the first O and the C
♦ There are 4 dots in this region. '4' indicates '2 pairs'
♦ So we put a ‘=’ between the first O and the C
♦ This is a double bond
• Consider the ‘shared region’ between the second O and the C
♦ There are 4 dots in this region. '4' indicates '2 pairs'
♦ So we put a ‘=’ between the second O and the C
♦ This is a double bond
■ So the shortened form is O=C=O
This is shown below the Lewis dot structure in fig.4.11(a)
2. Contribution from each atom
• Consider the ‘=’ between the first O and the C
♦ It is clear that:
✰ Two electrons in the '=' belongs to the O
✰ The remaining two electrons in the '=' belongs to the C
• Consider the ‘=’ between the second O and the C
♦ It is clear that:
✰ Two electrons in the '=' belongs to the O
✰ The remaining two electrons in the '=' belongs to the C
3. Checking the number of electrons in the Lewis dot structure:
• The first O has 8 dots around it
• The C has 8 dots around it
• The second O has 8 dots around it
4. The shortened form must show the 'lone pairs' also
• The C atom do not have any lone pairs
• The 'two lone pairs' of each O are indicated by four green dots around each O in the shortened form
Example 4:
• Fig.4.11(b) above shows the Lewis dot structure of C2H4
• The 1 valence electron of H is shown in green color
• The 4 valence electrons of C are shown in red color
• We can write the following 4 points:
1. Type of bond
• Consider the ‘shared region’ between the top-left H and the first C
♦ There are two dots in this region
♦ So we put a ‘─’ between this H and C
♦ This is a single bond
• Consider the ‘shared region’ between the bottom-left H and the first C
♦ There are two dots in this region
♦ So we put a ‘─’ between this H and C
♦ This is a single bond
• Consider the ‘shared region’ between the top-right H and the second C
♦ There are two dots in this region
♦ So we put a ‘─’ between this H and C
♦ This is a single bond
• Consider the ‘shared region’ between the bottom-right H and the second C
♦ There are two dots in this region
♦ So we put a ‘─’ between this H and C
♦ This is a single bond
• Consider the ‘shared region’ between the two C atoms
♦ There are 4 dots in this region. '4' indicates '2 pairs'
♦ So we put a ‘=’ between the two C atoms
♦ This is a double bond
■ The shortened form is shown in fig.4.11(c)
2. Contribution from each atom
• Consider the ‘─’ between the top-left H and the first C
♦ It is clear that:
✰ One electron in the '─' belongs to the H
✰ The other electron in the '─' belongs to the C
• Consider the ‘─’ between the bottom-left H and the first C
♦ It is clear that:
✰ One electron in the '─' belongs to the H
✰ The other electron in the '─' belongs to the C
• Consider the ‘─’ between the top-right H and the second C
♦ It is clear that:
✰ One electron in the '─' belongs to the H
✰ The other electron in the '─' belongs to the C
• Consider the ‘─’ between the bottom-right H and the second C
♦ It is clear that:
✰ One electron in the '─' belongs to the H
✰ The other electron in the '─' belongs to the C
• Consider the ‘=’ between the two C atoms
♦ It is clear that:
✰ Two electrons in the '=' belongs to the first C
✰ The remaining two electrons in the '=' belongs to the second C
3. Checking the number of electrons in the Lewis dot structure:
• Each of the four H atoms have 2 dots around them
• Each of the C atoms have 8 dots around them
(Remember that, H needs to attain duplet only)
4. The shortened form must show the 'lone pairs' also
• The C atoms do not have any lone pairs
• The H atoms also do not have any lone pairs
Example 5:
• Fig.4.12(a) below shows the Lewis dot structure of N2
 |
| Fig.4.12 |
• The 5 valence electrons of the second N are shown in green color
• We can write the following 4 points:
1. Type of bond
• Consider the ‘shared region’ between the two N atoms
♦ There are 6 dots in this region. '6' indicates '3 pairs'
♦ So we put a ‘☰’ between the two N s
♦ This is a triple bond
■ So the shortened form is N☰N
This is shown the Lewis dot structure in fig.4.12(a)
2. Contribution from each atom
• Consider the ‘☰’ between the two N atoms
♦ It is clear that:
✰ Three electrons in the '☰' belongs to the first N
✰ The remaining three electrons in the '☰' belongs to the second N
3. Checking the number of electrons in the Lewis dot structure:
• The first N has 8 dots around it
• The second N has 8 dots around it
4. The shortened form must show the 'lone pairs' also
• The 'one lone pair' of each N are indicated by two dots around each N in the shortened form
Example 6:
• Fig.4.12(b) above shows the Lewis dot structure of C2H2
• The 1 valence electron of H is shown in green color
• The 4 valence electrons of C are shown in red color
• The reader may write all the 4 points in his/her own note books as an exercise
• The above discussion will enable us to 'extract information' from any 'given Lewis dot structure'. In the next section, we will see the steps to draw Lewis dot structures of given molecules
No comments:
Post a Comment