In the previous section, we saw how to 'extract information' from any 'given Lewis dot structure'. In this section, we will see the steps to draw Lewis dot structures of 'given molecules'
We know that, a Lewis dot structure give us the following 3 information:
1. How atoms are bonded together in a molecule
2. How many pairs of electrons are shared between atoms
3. How is the octet attained by each atom in the molecule
• But what if we want to make a ‘new’ Lewis dot structure?
• Let us see the steps:
■ We will encounter two types:
(i) Polyatomic molecules
Examples: H2, CO2, H2O, NF3
(ii) Polyatomic ions
Examples: NH4+, CO32-
• First we will see polyatomic molecules
The following 6 steps will help us to make a Lewis dot structure of such molecules
Step 1: Finding the number of dots
• In a Lewis dot structure, we see a large number of dots
• So the first step will be to determine the answer to this question:
How many dots will be present in the final structure?
• The answer is simple:
All ‘available valence electrons’ will be present in the final structure
■ Let us see an example. We will write it in steps:
(i) Consider the molecule CH4
• C has the electronic configuration 1s22s22p2
♦ So it has 4 valence electrons
• H has the electronic configuration 1s1
♦ So it has 1 valence electron
(ii) So the total number of ‘available valence electrons’ = [4 + (4 × 1)] = 8
Thus, there will be 8 dots in the final structure
Step 2: The skeletal structure
• Draw the skeletal structure of the molecule
♦ The least electronegative atom will be the central atom
♦ The other atoms will be distributed around the central atom
An example:
• Consider the NF3 molecule
• N is less electronegative than F
(Electronegativity of N is 3.0 and that of F is 4.0)
♦ So N is the central atom
♦ The three F atoms will be distributed around the N atom
Step 3: Preliminary single bonds
• Connect the central atom to all the surrounding atoms with a single bond ('─')
• There will be at least one bond between atoms. So we can confidently put a ('─') at all possible connections
• The single bonds that we put in this step can be called preliminary bond
♦ This is because, in later steps, these single bonds may have to be changed to double or triple bonds
Step 4: Preliminary distribution of electrons
• Distribute all the 'available valence electrons' around the atoms
♦ Distribute in such a way that, the outer atoms get octet (duplet in case of H atoms) first
♦ Then give the remaining electrons to the central atom
• The distribution that we do in this step can be called preliminary distribution
♦ This is because, in later steps, this distribution may have to be changed
Step 5: Check for duplet and octet
• Check whether all H atoms (if present), have duplet
• Check whether all other atoms have octet
• If the required duplets and octets are not obtained, change the single bonds to double or triple bonds as necessary
• By the end of this step, the final structure should emerge
♦ All H atoms (if present), should be having duplet
♦ All other atoms should be having octet
Step 6: Check the number of dots
The total number of dots in the final structure must be same as the number obtained in step 1
Example 1: H2
Step 1: Finding the number of dots
• Number of valence electrons of H = 1
• So total number of valence electrons = (2 × 1) = 2
Step 2: The skeletal structure
• The skeletal structure is shown in fig.4.13(a) below:
Step 3: Preliminary single bonds
• The two H atoms are joined by a '─' as shown in fig.4.13(b) above
Step 4: Preliminary distribution of electrons
• Both are H atoms. So we need not look for the 'central atom' or 'outer atoms'
• Let us take the left side H atom
♦ We will give it duplet first
♦ And give the remaining electrons to the right side H atom
• This is shown in fig.4.13(c)
• In the fig.c, we see that:
♦ The valence electron of the 1st H atom are shown in green color
♦ The valence electron of the 2nd H atom are shown in red color
• The left side H atom has 2 electrons (including the one red dot in the single bond)
♦ The number of remaining electrons = (2-2) = 0
• There are no more electrons to distribute
Step 5: Check for duplet
• The 1st H atom has attained duplet
• The 2nd H atom has attained duplet
• So the '─' need not be changed to double or triple bonds
• Also, the preliminary distribution need not be changed
Step 6: Check the number of dots
♦ Total number of dots in fig.c = 2
♦ Number calculated in step 1 = 2
Example 2: NF3
Step 1: Finding the number of dots
• Number of valence electrons of N = 5
• Number of valence electrons of F = 7
• So total number of valence electrons = [5+(3 × 7)] = 26
Step 2: The skeletal structure
• N is less electronegative than F
(Electronegativity of N is 3.0 and that of F is 4.0)
♦ So N is the central atom
♦ The three F atoms will be distributed around the N atom
• The skeletal structure is shown in fig.4.14(a) below:
Step 3: Preliminary single bonds
• The four atoms are joined by a '─' as shown in fig.4.14(b) above
Step 4: Preliminary distribution of electrons
• First make the three outer F atoms octet
• Then give the remaining electrons to the central N atom
• This is shown in fig.c
• In the fig.c, we see that:
♦ The valence electrons of the F atoms are shown in green color
♦ The valence electrons of the N atom are shown in red color
• All the F atoms have 8 electrons (including the one red dot in the single bonds)
♦ So the F atoms use up (3 × 8) = 24 electrons
♦ The number of remaining electrons = (26-24) = 2
♦ These 2 electrons are given to the N atom
• There are no more electrons to distribute
Step 5: Check for octet
• Each of the F atoms have got 8 electrons. They have attained octet
• The N atom has got 8 electrons. It has attained octet
• Thus, the preliminary distribution need not be changed
Step 6: Check the number of dots
♦ Total number of dots in fig.c = 26
♦ Number calculated in step 1 = 26
Example 3: H2O
• We have already seen the finished structure of H2O in the previous section. Now we will see how that finished structure is obtained in steps:
Step 1: Finding the number of dots
• Number of valence electrons of H = 1
• Number of valence electrons of O = 6
• So total number of valence electrons = [(2 × 1)+6] = 8
Step 2: The skeletal structure
• H is less electronegative than O
(Electronegativity of H is 2.2 and that of O is 3.4)
♦ So H must be the central atom
♦ But there are two H atoms but only one O atom
♦ So we will make O the central atom
• The two H atoms will be distributed around the O atom
• The skeletal structure is shown in fig.4.15(a) below:
Step 3: Preliminary single bonds
• The three atoms are joined by a '─' as shown in fig.4.15(b) above
Step 4: Preliminary distribution of electrons
• First make the two outer H atoms duplet
• Then give the remaining electrons to the central O atom
• This is shown in fig.c
• In the fig.c, we see that:
♦ The valence electrons of the H atoms are shown in green color
♦ The valence electrons of the O atom are shown in red color
• All the H atoms have 2 electrons (including the one red dot in the single bonds)
♦ So the number of electrons used up for making the H atoms duplet = 4
♦ So the number of remaining electrons = (8-4) = 4
♦ These 4 electrons are given to the O atom
• There are no more electrons to distribute
Step 5: Check for octet
• Each of the H atoms have got 2 electrons. They have attained duplet
• The O atom has got 8 electrons. It has attained octet
• Thus, the preliminary distribution need not be changed
Step 6: Check the number of dots
♦ Total number of dots in fig.c = 8
♦ Number calculated in step 1 = 8
Example 4: O2
Step 1: Finding the number of dots
• Number of valence electrons of O = 6
• So total number of valence electrons = (2 × 6) = 12
Step 2: The skeletal structure
• The skeletal structure is shown in fig.4.16(a) below:
Step 3: Preliminary single bonds
• The two O atoms are joined by a '─' as shown in fig.4.16(b) above
Step 4: Preliminary distribution of electrons
• Both are O atoms. So we need not look for the 'central atom' or 'outer atoms'
• Let us take the left side O atom
♦ We will give it octet first
♦ And give the remaining electrons to the right side O atom
• This is shown in fig.4.16(c)
• In the fig.c, we see that:
♦ The valence electrons of the 1st O atom are shown in green color
♦ The valence electrons of the 2nd O atom are shown in red color
• The left side O atom has 8 electrons (including the one red dot in the single bond)
♦ The number of remaining electrons = (12-8) = 4
♦ These 4 electrons are given to the right side O atom
• There are no more electrons to distribute
Step 5: Check for octet
• The left side O atom has got 8 electrons
• The right side O atom has got only 6 electrons
♦ So this atom needs 2 more electrons
(i) Rearrangement: Change the preliminary single bond
♦ Change the single bond to double bond as shown in the fig.d
• Let us take the left side O atom
♦ We will give it octet first
♦ And give the remaining electrons to the right side O atom
• This is shown in fig.4.16(e)
• In the fig.e, we see that:
The left side O atom has 8 electrons (including the two red dots in the double bond)
♦ The number of remaining electrons = (12-8) = 4
♦ These 4 electrons are given to the right side O atom
• There are no more electrons to distribute
(ii) Check for octet:
♦ The left side O atom has 8 electrons
♦ The right side O atom has 8 electrons
Step 6: Check the number of dots
♦ Total number of dots in fig.e = 12
♦ Number calculated in step 1 = 12
Example 5: N2
Step 1: Finding the number of dots
• Number of valence electrons of N = 5
• So total number of valence electrons = (2 × 5) = 10
Step 2: The skeletal structure
• The skeletal structure is shown in fig.4.17(a) below:
Step 3: Preliminary single bonds
• The two N atoms are joined by a '─' as shown in fig.4.17(b) above
Step 4: Preliminary distribution of electrons
• Both are N atoms. So we need not look for the 'central atom' or 'outer atoms'
• Let us take the left side N atom
♦ We will give it octet first
♦ And give the remaining electrons to the right side N atom
• This is shown in fig.4.17(c)
• In the fig.c, we see that:
♦ The valence electrons of the 1st N atom are shown in green color
♦ The valence electrons of the 2nd N atom are shown in red color
• The left side N atom has 8 electrons (including the one red dot in the single bond)
♦ The number of remaining electrons = (10-8) = 2
♦ These 2 electrons are given to the right side N atom
• There are no more electrons to distribute
Step 5: Check for octet
• The left side N atom has got 8 electrons
• The right side N atom has got only 4 electrons
♦ So this atom needs 4 more electrons
(i) Rearrangement: Change the preliminary single bond
♦ Change the single bond to double bond as shown in the fig.d
• Let us take the left side N atom
♦ We will give it octet first
♦ And give the remaining electrons to the right side N atom
• This is shown in fig.4.17(e)
• In the fig.e, we see that:
The left side N atom has 8 electrons (including the two red dots in the double bond)
♦ The number of remaining electrons = (10-8) = 2
♦ These 2 electrons are given to the right side N atom
• There are no more electrons to distribute
(ii) Check for octet:
• The left side N atom has got 8 electrons
• The right side N atom has got only 6 electrons
♦ So this atom needs 2 more electrons
(i) Rearrangement: Change the double bond
♦ Change the double bond to triple bond as shown in the fig.f
• Let us take the left side N atom
♦ We will give it octet first
♦ And give the remaining electrons to the right side N atom
• This is shown in fig.4.17(g)
• In the fig.g, we see that:
The left side N atom has 8 electrons (including the three red dots in the triple bond)
♦ The number of remaining electrons = (10-8) = 2
♦ These 2 electrons are given to the right side N atom
• There are no more electrons to distribute
(ii) Check for octet:
♦ The left side N atom has 8 electrons
♦ The right side N atom has 8 electrons
Step 6: Check the number of dots
♦ Total number of dots in fig.g = 10
♦ Number calculated in step 1 = 10
Example 6: CO
Step 1: Finding the number of dots
• Number of valence electrons of C = 4
• Number of valence electrons of O = 6
• So total number of valence electrons = (4+6) = 10
Step 2: The skeletal structure
• The skeletal structure is shown in fig.4.18(a) below:
Step 3: Preliminary single bonds
• The two atoms are joined by a '─' as shown in fig.4.18(b) above
Step 4: Preliminary distribution of electrons
• There are only two atoms. So we need not look for the 'central atom' or 'outer atoms'
• Let us take the C atom
♦ We will give it octet first
♦ And give the remaining electrons to the O atom
• This is shown in fig.4.18(c)
• In the fig.c, we see that:
♦ The valence electrons of the C atom are shown in green color
♦ The valence electrons of the O atom are shown in red color
• The C atom has 8 electrons (including the one red dot in the single bond)
♦ The number of remaining electrons = (10-8) = 2
♦ These 2 electrons are given to the O atom
• There are no more electrons to distribute
Step 5: Check for octet
• The C atom has got 8 electrons
• The O atom has got only 4 electrons
♦ So this atom needs 4 more electrons
(i) Rearrangement: Change the preliminary single bond
♦ Change the single bond to double bond as shown in the fig.d
• Let us take the C atom
♦ We will give it octet first
♦ And give the remaining electrons to the O atom
• This is shown in fig.4.18(e)
• In the fig.e, we see that:
The C atom has 8 electrons (including the two red dots in the double bond)
♦ The number of remaining electrons = (10-8) = 2
♦ These 2 electrons are given to the O atom
• There are no more electrons to distribute
(ii) Check for octet:
• The C atom has got 8 electrons
• The O atom has got only 6 electrons
♦ So this atom needs 2 more electrons
(i) Rearrangement: Change the double bond
♦ Change the double bond to triple bond as shown in the fig.f
• Let us take the C atom
♦ We will give it octet first
♦ And give the remaining electrons to the O atom
• This is shown in fig.4.18(g)
• In the fig.g, we see that:
The C atom has 8 electrons (including the three red dots in the triple bond)
♦ The number of remaining electrons = (10-8) = 2
♦ These 2 electrons are given to the O atom
• There are no more electrons to distribute
(ii) Check for octet:
♦ The C atom has 8 electrons
♦ The O atom has 8 electrons
Step 6: Check the number of dots
♦ Total number of dots in fig.g = 10
♦ Number calculated in step 1 = 10
We know that, a Lewis dot structure give us the following 3 information:
1. How atoms are bonded together in a molecule
2. How many pairs of electrons are shared between atoms
3. How is the octet attained by each atom in the molecule
• But what if we want to make a ‘new’ Lewis dot structure?
• Let us see the steps:
■ We will encounter two types:
(i) Polyatomic molecules
Examples: H2, CO2, H2O, NF3
(ii) Polyatomic ions
Examples: NH4+, CO32-
• First we will see polyatomic molecules
The following 6 steps will help us to make a Lewis dot structure of such molecules
Step 1: Finding the number of dots
• In a Lewis dot structure, we see a large number of dots
• So the first step will be to determine the answer to this question:
How many dots will be present in the final structure?
• The answer is simple:
All ‘available valence electrons’ will be present in the final structure
■ Let us see an example. We will write it in steps:
(i) Consider the molecule CH4
• C has the electronic configuration 1s22s22p2
♦ So it has 4 valence electrons
• H has the electronic configuration 1s1
♦ So it has 1 valence electron
(ii) So the total number of ‘available valence electrons’ = [4 + (4 × 1)] = 8
Thus, there will be 8 dots in the final structure
Step 2: The skeletal structure
• Draw the skeletal structure of the molecule
♦ The least electronegative atom will be the central atom
♦ The other atoms will be distributed around the central atom
An example:
• Consider the NF3 molecule
• N is less electronegative than F
(Electronegativity of N is 3.0 and that of F is 4.0)
♦ So N is the central atom
♦ The three F atoms will be distributed around the N atom
Step 3: Preliminary single bonds
• Connect the central atom to all the surrounding atoms with a single bond ('─')
• There will be at least one bond between atoms. So we can confidently put a ('─') at all possible connections
• The single bonds that we put in this step can be called preliminary bond
♦ This is because, in later steps, these single bonds may have to be changed to double or triple bonds
Step 4: Preliminary distribution of electrons
• Distribute all the 'available valence electrons' around the atoms
♦ Distribute in such a way that, the outer atoms get octet (duplet in case of H atoms) first
♦ Then give the remaining electrons to the central atom
• The distribution that we do in this step can be called preliminary distribution
♦ This is because, in later steps, this distribution may have to be changed
Step 5: Check for duplet and octet
• Check whether all H atoms (if present), have duplet
• Check whether all other atoms have octet
• If the required duplets and octets are not obtained, change the single bonds to double or triple bonds as necessary
• By the end of this step, the final structure should emerge
♦ All H atoms (if present), should be having duplet
♦ All other atoms should be having octet
Step 6: Check the number of dots
The total number of dots in the final structure must be same as the number obtained in step 1
Let us now apply the above rules to some molecules:
Step 1: Finding the number of dots
• Number of valence electrons of H = 1
• So total number of valence electrons = (2 × 1) = 2
Step 2: The skeletal structure
• The skeletal structure is shown in fig.4.13(a) below:
 |
| Fig.4.13 |
• The two H atoms are joined by a '─' as shown in fig.4.13(b) above
Step 4: Preliminary distribution of electrons
• Both are H atoms. So we need not look for the 'central atom' or 'outer atoms'
• Let us take the left side H atom
♦ We will give it duplet first
♦ And give the remaining electrons to the right side H atom
• This is shown in fig.4.13(c)
• In the fig.c, we see that:
♦ The valence electron of the 1st H atom are shown in green color
♦ The valence electron of the 2nd H atom are shown in red color
• The left side H atom has 2 electrons (including the one red dot in the single bond)
♦ The number of remaining electrons = (2-2) = 0
• There are no more electrons to distribute
Step 5: Check for duplet
• The 1st H atom has attained duplet
• The 2nd H atom has attained duplet
• So the '─' need not be changed to double or triple bonds
• Also, the preliminary distribution need not be changed
Step 6: Check the number of dots
♦ Total number of dots in fig.c = 2
♦ Number calculated in step 1 = 2
Example 2: NF3
Step 1: Finding the number of dots
• Number of valence electrons of N = 5
• Number of valence electrons of F = 7
• So total number of valence electrons = [5+(3 × 7)] = 26
Step 2: The skeletal structure
• N is less electronegative than F
(Electronegativity of N is 3.0 and that of F is 4.0)
♦ So N is the central atom
♦ The three F atoms will be distributed around the N atom
• The skeletal structure is shown in fig.4.14(a) below:
 |
| Fig.4.14 |
• The four atoms are joined by a '─' as shown in fig.4.14(b) above
Step 4: Preliminary distribution of electrons
• First make the three outer F atoms octet
• Then give the remaining electrons to the central N atom
• This is shown in fig.c
• In the fig.c, we see that:
♦ The valence electrons of the F atoms are shown in green color
♦ The valence electrons of the N atom are shown in red color
• All the F atoms have 8 electrons (including the one red dot in the single bonds)
♦ So the F atoms use up (3 × 8) = 24 electrons
♦ The number of remaining electrons = (26-24) = 2
♦ These 2 electrons are given to the N atom
• There are no more electrons to distribute
Step 5: Check for octet
• Each of the F atoms have got 8 electrons. They have attained octet
• The N atom has got 8 electrons. It has attained octet
• Thus, the preliminary distribution need not be changed
Step 6: Check the number of dots
♦ Total number of dots in fig.c = 26
♦ Number calculated in step 1 = 26
Example 3: H2O
• We have already seen the finished structure of H2O in the previous section. Now we will see how that finished structure is obtained in steps:
Step 1: Finding the number of dots
• Number of valence electrons of H = 1
• Number of valence electrons of O = 6
• So total number of valence electrons = [(2 × 1)+6] = 8
Step 2: The skeletal structure
• H is less electronegative than O
(Electronegativity of H is 2.2 and that of O is 3.4)
♦ So H must be the central atom
♦ But there are two H atoms but only one O atom
♦ So we will make O the central atom
• The two H atoms will be distributed around the O atom
• The skeletal structure is shown in fig.4.15(a) below:
 |
| Fig.4.15 |
• The three atoms are joined by a '─' as shown in fig.4.15(b) above
Step 4: Preliminary distribution of electrons
• First make the two outer H atoms duplet
• Then give the remaining electrons to the central O atom
• This is shown in fig.c
• In the fig.c, we see that:
♦ The valence electrons of the H atoms are shown in green color
♦ The valence electrons of the O atom are shown in red color
• All the H atoms have 2 electrons (including the one red dot in the single bonds)
♦ So the number of electrons used up for making the H atoms duplet = 4
♦ So the number of remaining electrons = (8-4) = 4
♦ These 4 electrons are given to the O atom
• There are no more electrons to distribute
Step 5: Check for octet
• Each of the H atoms have got 2 electrons. They have attained duplet
• The O atom has got 8 electrons. It has attained octet
• Thus, the preliminary distribution need not be changed
Step 6: Check the number of dots
♦ Total number of dots in fig.c = 8
♦ Number calculated in step 1 = 8
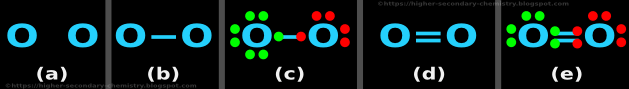
Example 4: O2
Step 1: Finding the number of dots
• Number of valence electrons of O = 6
• So total number of valence electrons = (2 × 6) = 12
Step 2: The skeletal structure
• The skeletal structure is shown in fig.4.16(a) below:
 |
| Fig.4.16 |
• The two O atoms are joined by a '─' as shown in fig.4.16(b) above
Step 4: Preliminary distribution of electrons
• Both are O atoms. So we need not look for the 'central atom' or 'outer atoms'
• Let us take the left side O atom
♦ We will give it octet first
♦ And give the remaining electrons to the right side O atom
• This is shown in fig.4.16(c)
• In the fig.c, we see that:
♦ The valence electrons of the 1st O atom are shown in green color
♦ The valence electrons of the 2nd O atom are shown in red color
• The left side O atom has 8 electrons (including the one red dot in the single bond)
♦ The number of remaining electrons = (12-8) = 4
♦ These 4 electrons are given to the right side O atom
• There are no more electrons to distribute
Step 5: Check for octet
• The left side O atom has got 8 electrons
• The right side O atom has got only 6 electrons
♦ So this atom needs 2 more electrons
(i) Rearrangement: Change the preliminary single bond
♦ Change the single bond to double bond as shown in the fig.d
• Let us take the left side O atom
♦ We will give it octet first
♦ And give the remaining electrons to the right side O atom
• This is shown in fig.4.16(e)
• In the fig.e, we see that:
The left side O atom has 8 electrons (including the two red dots in the double bond)
♦ The number of remaining electrons = (12-8) = 4
♦ These 4 electrons are given to the right side O atom
• There are no more electrons to distribute
(ii) Check for octet:
♦ The left side O atom has 8 electrons
♦ The right side O atom has 8 electrons
Step 6: Check the number of dots
♦ Total number of dots in fig.e = 12
♦ Number calculated in step 1 = 12
Example 5: N2
Step 1: Finding the number of dots
• Number of valence electrons of N = 5
• So total number of valence electrons = (2 × 5) = 10
Step 2: The skeletal structure
• The skeletal structure is shown in fig.4.17(a) below:
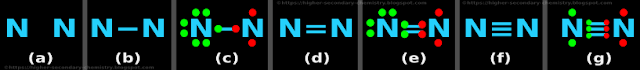 |
| Fig.4.17 |
• The two N atoms are joined by a '─' as shown in fig.4.17(b) above
Step 4: Preliminary distribution of electrons
• Both are N atoms. So we need not look for the 'central atom' or 'outer atoms'
• Let us take the left side N atom
♦ We will give it octet first
♦ And give the remaining electrons to the right side N atom
• This is shown in fig.4.17(c)
• In the fig.c, we see that:
♦ The valence electrons of the 1st N atom are shown in green color
♦ The valence electrons of the 2nd N atom are shown in red color
• The left side N atom has 8 electrons (including the one red dot in the single bond)
♦ The number of remaining electrons = (10-8) = 2
♦ These 2 electrons are given to the right side N atom
• There are no more electrons to distribute
Step 5: Check for octet
• The left side N atom has got 8 electrons
• The right side N atom has got only 4 electrons
♦ So this atom needs 4 more electrons
(i) Rearrangement: Change the preliminary single bond
♦ Change the single bond to double bond as shown in the fig.d
• Let us take the left side N atom
♦ We will give it octet first
♦ And give the remaining electrons to the right side N atom
• This is shown in fig.4.17(e)
• In the fig.e, we see that:
The left side N atom has 8 electrons (including the two red dots in the double bond)
♦ The number of remaining electrons = (10-8) = 2
♦ These 2 electrons are given to the right side N atom
• There are no more electrons to distribute
(ii) Check for octet:
• The left side N atom has got 8 electrons
• The right side N atom has got only 6 electrons
♦ So this atom needs 2 more electrons
(i) Rearrangement: Change the double bond
♦ Change the double bond to triple bond as shown in the fig.f
• Let us take the left side N atom
♦ We will give it octet first
♦ And give the remaining electrons to the right side N atom
• This is shown in fig.4.17(g)
• In the fig.g, we see that:
The left side N atom has 8 electrons (including the three red dots in the triple bond)
♦ The number of remaining electrons = (10-8) = 2
♦ These 2 electrons are given to the right side N atom
• There are no more electrons to distribute
(ii) Check for octet:
♦ The left side N atom has 8 electrons
♦ The right side N atom has 8 electrons
Step 6: Check the number of dots
♦ Total number of dots in fig.g = 10
♦ Number calculated in step 1 = 10
Example 6: CO
Step 1: Finding the number of dots
• Number of valence electrons of C = 4
• Number of valence electrons of O = 6
• So total number of valence electrons = (4+6) = 10
Step 2: The skeletal structure
• The skeletal structure is shown in fig.4.18(a) below:
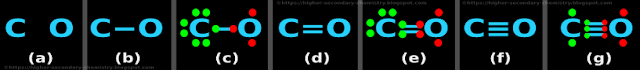 |
| Fig.4.18 |
• The two atoms are joined by a '─' as shown in fig.4.18(b) above
Step 4: Preliminary distribution of electrons
• There are only two atoms. So we need not look for the 'central atom' or 'outer atoms'
• Let us take the C atom
♦ We will give it octet first
♦ And give the remaining electrons to the O atom
• This is shown in fig.4.18(c)
• In the fig.c, we see that:
♦ The valence electrons of the C atom are shown in green color
♦ The valence electrons of the O atom are shown in red color
• The C atom has 8 electrons (including the one red dot in the single bond)
♦ The number of remaining electrons = (10-8) = 2
♦ These 2 electrons are given to the O atom
• There are no more electrons to distribute
Step 5: Check for octet
• The C atom has got 8 electrons
• The O atom has got only 4 electrons
♦ So this atom needs 4 more electrons
(i) Rearrangement: Change the preliminary single bond
♦ Change the single bond to double bond as shown in the fig.d
• Let us take the C atom
♦ We will give it octet first
♦ And give the remaining electrons to the O atom
• This is shown in fig.4.18(e)
• In the fig.e, we see that:
The C atom has 8 electrons (including the two red dots in the double bond)
♦ The number of remaining electrons = (10-8) = 2
♦ These 2 electrons are given to the O atom
• There are no more electrons to distribute
(ii) Check for octet:
• The C atom has got 8 electrons
• The O atom has got only 6 electrons
♦ So this atom needs 2 more electrons
(i) Rearrangement: Change the double bond
♦ Change the double bond to triple bond as shown in the fig.f
• Let us take the C atom
♦ We will give it octet first
♦ And give the remaining electrons to the O atom
• This is shown in fig.4.18(g)
• In the fig.g, we see that:
The C atom has 8 electrons (including the three red dots in the triple bond)
♦ The number of remaining electrons = (10-8) = 2
♦ These 2 electrons are given to the O atom
• There are no more electrons to distribute
(ii) Check for octet:
♦ The C atom has 8 electrons
♦ The O atom has 8 electrons
Step 6: Check the number of dots
♦ Total number of dots in fig.g = 10
♦ Number calculated in step 1 = 10
Some more examples are given below:
1. CCl4 Carbon tetra chloride
2. SiCl4 Silicon tetra chloride
3. H2S Hydrogen sulfide
1. CCl4 Carbon tetra chloride
2. SiCl4 Silicon tetra chloride
3. H2S Hydrogen sulfide
• The above discussion will enable us to 'draw Lewis dot structures' of some polyatomic molecules
• In the next section, we will see the steps to draw Lewis dot structures of given polyatomic ions
• In the next section, we will see the steps to draw Lewis dot structures of given polyatomic ions
No comments:
Post a Comment