In the previous section 3.11, we completed the chapter on classification of elements and periodic trends. In this chapter, we will see chemical bonding and molecular structure
1. Taking two samples:
■ We know that, ‘any thing which occupies space’ is called matter
• So consider two samples (Sample A and Sample B) of ‘two different things that occupy space’
♦ Suppose that, ‘Sample A’ is a sample of any one of the noble gases
♦ Suppose that, ‘Sample B’ does not contain any one of the noble gases
2. Then we can write two points:
(i) Sample A will contain independent atoms
(ii) Sample B will not contain even a single independent atom
■ How can we be so sure about Sample B?
• Answer can be written in just one sentence:
No element (except noble gases) can exist as independent atoms
3. So in what form do the ‘elements other than noble gases’ exist?
• The answer can be written in 2 steps:
(i) ‘Elements other than noble gases’ exist as independent molecules
(ii) Each molecule will contain two or more atoms
• In some cases, ‘those atoms in a molecule’ will be of the same type
♦ For example, in the molecule O2, all are O atoms
• In some cases, ‘those atoms in a molecule’ will be of different types
♦ For example, in the molecule H2O, there are H and O atoms
4. Then the next question arises:
■ How do ‘those atoms in a molecule’ stick together?
• The answer can be written in steps:
(i) There exists a ‘force of attraction’ between individual atoms in a molecule
(ii) Due to the presence of this 'attractive force', the atoms cannot separate away from each other
(iii) This 'attractive force between atoms' is called chemical bond
■ We can write the definition in a single sentence:
The attractive force which holds various constituents (atoms, ions, etc.) together in different chemical species is called a chemical bond
5. So it is clear that, individual atoms of various elements combine together to form a molecule
■ But this information leads to several more questions:
• Why do atoms combine?
• Why are only certain combinations possible?
• Why do some atoms combine while certain others do not?
• Why do molecules possess definite shapes?
In this chapter we will try to find the answers to these questions
• This model gave a basic explanation of 'how various atoms stick together in a molecule'
• G. N. Lewis was an American scientist
• Walther Kossel was a German scientist
• Though the model is known as Kossel-Lewis model, the two scientists had worked independently
• Let us write the salient features of this model:
Feature 1. Parts of an atom
• Consider an atom
• It will consist of two parts:
(i) An inner ‘kernel’
♦ The 'kernel' is positively charged
♦ But it consists of the nucleus as well as the inner electrons
(ii) The 'outer shell'
♦ The 'outer shell' is the shell which contains the outermost electrons (valence electrons)
Let us see some examples:
Example 1:
• The electronic configuration of Na is 1s22s22p63s1
♦ This is same as [Ne]3s1
• The 'kernel' of Na will consist of two items:
♦ The nucleus of Na
♦ The electrons of [Ne]
• The 'outer shell' of Na will be the 'shell which contains the last single electron'
• The 'kernel' and the 'outer shell' together constitute the atom
Example 2:
• The electronic configuration of Cl is 1s22s22p63s23p5
♦ This is same as [Ne]3s23p5
• The 'kernel' of Cl will consist of two items:
♦ The nucleus of Cl
♦ The electrons of [Ne]
• The 'outer shell' of Cl will be the 'shell which contains the last 7 electrons'
• The 'kernel' and the 'outer shell' together constitute the atom
Feature 2: The number of electrons in the 'outer shell'
• There can be a maximum of 8 electrons in the 'outer shell'
Feature 3: Shape of the 'outer shell'
• The 'outer shell' is in the shape of a cube
• This cube surrounds the 'kernel'
• This is shown in fig.4.1 below:
Feature 4: Positions of the electrons in the 'outer shell'
• The electrons are situated at the corners of the cube
• Any cube will have 8 corners
• So the 'outer shell' can accommodate a maximum of 8 electrons
Examples:
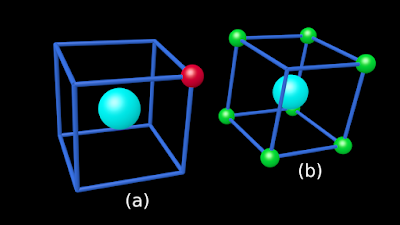
♦ The one and only outer electron of Na will be situated at one of the total 8 corners
✰ This is shown in fig.4.2(a) below
✰ Note that, 7 corners of the cube are vacant
♦ The 7 outer electrons of Cl will be situated at 7 of the total 8 corners
✰ This is shown in fig.4.2(b) below
✰ Note that, only 1 corner of the cube is vacant
Feature 5: Arrangement in the case of noble gases
• In the case of noble gases, all the 8 corners will be occupied
• When all the 8 corners are occupied, we say this: The atom has attained octet
■ An atom which has attained octet is stable
• In other words, the atom which has octet, has a stable electronic configuration
Feature 6: Octet of atoms other than noble gases
• Atoms other than noble gases try to attain octet
• They attain octet by 'entering into chemical bonds' with other atoms
Feature 7: The two methods for 'entering into chemical bonds'
Method 1:
• One or more electrons will be transferred from one atom to the other atom
♦ After the transfer, 'the atom which loses electrons' will be having 8 electrons at the 8 corners
♦ After the transfer, 'the atom which gains electrons' also will be having 8 electrons at the 8 corners
An example:
• Na loses it's one and only electron and becomes Na+
♦ As a result, [Ne]3s1 becomes [Ne]
♦ [Ne] has 8 electrons in the outermost shell
• Cl gains the 'electron lost by Na' and becomes Cl-
♦ As a result, [Ne]3s23p5 becomes [Ne]3s23p6
♦ [Ne]3s23p6 has 8 electrons in the outermost shell
• Na+ is positively charged and Cl- is negatively charged
♦ As a result, an electrostatic force of attraction comes into effect between the two ions
♦ So we will not be able to separate the two ions from each other
♦ This is shown in fig.4.3 below:
Method 2:
• A 'pair of electrons' (two electrons) is shared between two atoms
• When two electrons are shared in this way, both the atoms will be having 8 electrons at the respective 8 corners
An example:
• Fig.4.4 below shows two independent Cl atoms
• The 7 outermost electrons of the first Cl atom are shown in red color
• The 7 outermost electrons of the second Cl atom are shown in green color
• Make a note of the electron marked as 'A' in the first Cl atom
♦ It has an adjacent vacant corner
• Make a note of the electron marked as 'B' in the first Cl atom
♦ It also has an adjacent vacant corner
• Now consider fig.4.5 below:
• The two Cl atoms are now combined to form a Cl molecule
♦ The electron 'A' occupies the corner which was vacant in the second Cl atom
♦ The electron 'B' occupies the corner which was vacant in the first Cl atom
■ From the view point of the first Cl atom, all it's 8 corners are now occupied
♦ Thus this Cl atom has attained octet
■ From the view point of the second Cl atom, all it's 8 corners are now occupied
♦ Thus this Cl atom also has attained octet
• Electrons 'A' and 'B' constitute the 'shared pair'
♦ Both the Cl atoms have equal claim on both 'A' and 'B'
♦ So the two Cl atoms cannot move away from each other
■ 'Both the electrons in the pair' belongs to both the atoms
Feature 8: Using symbols
• It is not easy to draw 3D models of the cube for every atoms
• So Lewis developed a simplified method
• Only the electrons in the outer shell will take part in chemical reactions
• The electrons in the inner shells are well protected. In most cases, they do not take part in chemical reactions
■ Lewis noticed that, we need to show the outermost electrons only
♦ There electrons are shown as dots
♦ The dots are marked around the ‘symbol of atoms’
■ This notation is called Lewis symbol
• The fig.4.6 below shows the Lewis symbols of elements of the 2nd period
Feature 9: Significance of Lewis symbols
• The ‘number of dots’ in the Lewis symbol can be used to calculate the common valence or group valence
• When the number of dots is less than or equal to 4:
♦ Common valence = Number of dots
• When the number of dots is greater than 4:
♦ Common valence = 8 – number of dots
• In addition to the above, Kossel gave a few more information. They are known as Kossel's Postulates
(A postulate is 'something which is assumed to be true'. So that, it can be used as a basis for reasoning or discussion. The dictionary meaning can be seen here)
• They can be written in 6 steps
1. We know that:
• Alkali metals (group 1) are highly electropositive
• Halogens (group 17) are highly electronegative
2. Also we know that, in the periodic table,
• Alkali metals are at the left end
• Halogens are near the right end
3. Alkali metals being electropositive, readily lose their outermost single electron
• When that electron is lost, the atom becomes a +ve ion
♦ The +ve ion thus formed will be having the electronic configuration of a noble gas
♦ ‘Electronic configuration of a noble gas’ is a very stable configuration
♦ That means, the +ve ion formed from the ‘alkali metal atom’ will be very stable
4. Halogens being electronegative, readily accepts one more electron
• When that electron is gained, the atom becomes an -ve ion
♦ The -ve ion thus formed will be having the electronic configuration of a noble gas
♦ ‘Electronic configuration of a noble gas’ is a very stable configuration
♦ That means, the -ve ion formed from the ‘halogen atom’ will be very stable
5. So we have a 'stable +ve ion' and a 'stable -ve ion'
• An electrostatic force of attraction comes into play between the two oppositely charged ions
• Due to this electrostatic force of attraction, we will not be able to separate the two ions
♦ The two ions will always stick together
♦ Thus a molecule is formed
• We will see two examples:
Example 1: Formation of sodium chloride (NaCl)
• Na is an alkali metal
• It loses one electron as shown below:
$\mathbf\small{\rm{Na\longrightarrow Na^{+}+e^{-}}}$
♦ This is same as [Ne]3s1 becoming [Ne]
• Cl is a halogen
• It gains one electron as shown below:
$\mathbf\small{\rm{Cl+e^{-}\longrightarrow Cl^{-}}}$
♦ This is same as [Ne]3s23p5 becoming [Ne]3s23p6 or [Ar]
■ The Na+ and Cl- thus formed will stick together (due to electrostatic force of attraction) as shown below:
$\mathbf\small{\rm{Na^{+}+Cl^{-}\longrightarrow NaCl\,\,\;OR\;\,\,Na^{+}Cl^{-}}}$
• The fig.4.7 below shows the above result using Lewis symbols:
Example 2: Formation of calcium fluoride (CaF2)
• Ca is an alkaline earth metal (group 2)
• It loses two electrons as shown below:
$\mathbf\small{\rm{Ca\longrightarrow Ca^{2+}+2e^{-}}}$
♦ This is same as [Ar]4s2 becoming [Ar]
• F is a halogen
• It gains one electron as shown below:
$\mathbf\small{\rm{F+e^{-}\longrightarrow F^{-}}}$
♦ This is same as [He]2s22p5 becoming [He]2s22p6 or [Ne]
■ The Ca+ and 2F- thus formed will stick together (due to electrostatic force of attraction) as shown below:
$\mathbf\small{\rm{Ca^{2+}+2F^{-}\longrightarrow CaF_2\,\,\;OR\;\,\,Ca^{2+}(F^{-})_2}}$
• The fig.4.8 below shows the above result using Lewis symbols:
■ Note:
♦ One Ca atom loses two electrons
♦ But one F atom can accept only one electron
♦ So 'two F atoms' will be required to accept 'the two electrons' lost by 'the one Ca'
6. In the above two examples, we see a 'chemical bonding' between two ions
• This chemical bonding helps in the formation of a molecule
• This chemical bonding is possible because of the electrostatic force of attraction between +ve and -ve ions
■ So Kossel called it: electrovalent bond
■ We can write the definition in one sentence:
The bond formed, as a result of the electrostatic attraction between the positive and negative ions was termed (by Kossel) as electrovalent bond
■ Kossel gave the definition for electrovalence also:
The charge possessed by an ion, when that ion is part of an electrovalent bond is called electrovalence
Some examples:
♦ Electrovalence of Na is +1
♦ Electrovalence of Ca is +2
♦ Electrovalence of Cl is -1
♦ Electrovalence of F is -1
■ Kossel's Postulates provided a strong foundation for further studies about 'structure of ionic compounds'. However, Kossel and other scientists of that time knew that, the 'structures of a large number of compounds' cannot be explained using these postulates. We will see them in later sections
Write Lewis dot symbols for the atoms of the following elements:
Mg. Na, B, O, N, Br
Solution:
The required Lewis dot symbols are shown in fig. below:
Sample explanation:
• Consider Br. It has the electronic configuration: 1s22s22p23s23p63d104s24p5 OR [Ar]3d104s24p5
• So the outermost main-shell has 7 electrons. That means, there are 7 valence electrons
• Thus there will be 7 dots in the Lewis dot symbol of Br
1. Taking two samples:
■ We know that, ‘any thing which occupies space’ is called matter
• So consider two samples (Sample A and Sample B) of ‘two different things that occupy space’
♦ Suppose that, ‘Sample A’ is a sample of any one of the noble gases
♦ Suppose that, ‘Sample B’ does not contain any one of the noble gases
2. Then we can write two points:
(i) Sample A will contain independent atoms
(ii) Sample B will not contain even a single independent atom
■ How can we be so sure about Sample B?
• Answer can be written in just one sentence:
No element (except noble gases) can exist as independent atoms
3. So in what form do the ‘elements other than noble gases’ exist?
• The answer can be written in 2 steps:
(i) ‘Elements other than noble gases’ exist as independent molecules
(ii) Each molecule will contain two or more atoms
• In some cases, ‘those atoms in a molecule’ will be of the same type
♦ For example, in the molecule O2, all are O atoms
• In some cases, ‘those atoms in a molecule’ will be of different types
♦ For example, in the molecule H2O, there are H and O atoms
4. Then the next question arises:
■ How do ‘those atoms in a molecule’ stick together?
• The answer can be written in steps:
(i) There exists a ‘force of attraction’ between individual atoms in a molecule
(ii) Due to the presence of this 'attractive force', the atoms cannot separate away from each other
(iii) This 'attractive force between atoms' is called chemical bond
■ We can write the definition in a single sentence:
The attractive force which holds various constituents (atoms, ions, etc.) together in different chemical species is called a chemical bond
5. So it is clear that, individual atoms of various elements combine together to form a molecule
■ But this information leads to several more questions:
• Why do atoms combine?
• Why are only certain combinations possible?
• Why do some atoms combine while certain others do not?
• Why do molecules possess definite shapes?
In this chapter we will try to find the answers to these questions
• In the year 1916 Kossel and Lewis succeeded in presenting a satisfactory 'model of molecule'
• G. N. Lewis was an American scientist
• Walther Kossel was a German scientist
• Though the model is known as Kossel-Lewis model, the two scientists had worked independently
• Let us write the salient features of this model:
Feature 1. Parts of an atom
• Consider an atom
• It will consist of two parts:
(i) An inner ‘kernel’
♦ The 'kernel' is positively charged
♦ But it consists of the nucleus as well as the inner electrons
(ii) The 'outer shell'
♦ The 'outer shell' is the shell which contains the outermost electrons (valence electrons)
Let us see some examples:
Example 1:
• The electronic configuration of Na is 1s22s22p63s1
♦ This is same as [Ne]3s1
• The 'kernel' of Na will consist of two items:
♦ The nucleus of Na
♦ The electrons of [Ne]
• The 'outer shell' of Na will be the 'shell which contains the last single electron'
• The 'kernel' and the 'outer shell' together constitute the atom
Example 2:
• The electronic configuration of Cl is 1s22s22p63s23p5
♦ This is same as [Ne]3s23p5
• The 'kernel' of Cl will consist of two items:
♦ The nucleus of Cl
♦ The electrons of [Ne]
• The 'outer shell' of Cl will be the 'shell which contains the last 7 electrons'
• The 'kernel' and the 'outer shell' together constitute the atom
Feature 2: The number of electrons in the 'outer shell'
• There can be a maximum of 8 electrons in the 'outer shell'
Feature 3: Shape of the 'outer shell'
• The 'outer shell' is in the shape of a cube
• This cube surrounds the 'kernel'
• This is shown in fig.4.1 below:
 |
| Fig.4.1 |
• The electrons are situated at the corners of the cube
• Any cube will have 8 corners
• So the 'outer shell' can accommodate a maximum of 8 electrons
Examples:
♦ The one and only outer electron of Na will be situated at one of the total 8 corners
✰ This is shown in fig.4.2(a) below
✰ Note that, 7 corners of the cube are vacant
♦ The 7 outer electrons of Cl will be situated at 7 of the total 8 corners
✰ This is shown in fig.4.2(b) below
✰ Note that, only 1 corner of the cube is vacant
 |
| Fig.4.2 |
• In the case of noble gases, all the 8 corners will be occupied
• When all the 8 corners are occupied, we say this: The atom has attained octet
■ An atom which has attained octet is stable
• In other words, the atom which has octet, has a stable electronic configuration
Feature 6: Octet of atoms other than noble gases
• Atoms other than noble gases try to attain octet
• They attain octet by 'entering into chemical bonds' with other atoms
Feature 7: The two methods for 'entering into chemical bonds'
Method 1:
• One or more electrons will be transferred from one atom to the other atom
♦ After the transfer, 'the atom which loses electrons' will be having 8 electrons at the 8 corners
♦ After the transfer, 'the atom which gains electrons' also will be having 8 electrons at the 8 corners
An example:
• Na loses it's one and only electron and becomes Na+
♦ As a result, [Ne]3s1 becomes [Ne]
♦ [Ne] has 8 electrons in the outermost shell
• Cl gains the 'electron lost by Na' and becomes Cl-
♦ As a result, [Ne]3s23p5 becomes [Ne]3s23p6
♦ [Ne]3s23p6 has 8 electrons in the outermost shell
• Na+ is positively charged and Cl- is negatively charged
♦ As a result, an electrostatic force of attraction comes into effect between the two ions
♦ So we will not be able to separate the two ions from each other
♦ This is shown in fig.4.3 below:
 |
| Fig.4.3 |
• A 'pair of electrons' (two electrons) is shared between two atoms
• When two electrons are shared in this way, both the atoms will be having 8 electrons at the respective 8 corners
An example:
• Fig.4.4 below shows two independent Cl atoms
 |
| Fig.4.4 |
• The 7 outermost electrons of the second Cl atom are shown in green color
• Make a note of the electron marked as 'A' in the first Cl atom
♦ It has an adjacent vacant corner
• Make a note of the electron marked as 'B' in the first Cl atom
♦ It also has an adjacent vacant corner
• Now consider fig.4.5 below:
 |
| Fig.4.5 |
♦ The electron 'A' occupies the corner which was vacant in the second Cl atom
♦ The electron 'B' occupies the corner which was vacant in the first Cl atom
■ From the view point of the first Cl atom, all it's 8 corners are now occupied
♦ Thus this Cl atom has attained octet
■ From the view point of the second Cl atom, all it's 8 corners are now occupied
♦ Thus this Cl atom also has attained octet
• Electrons 'A' and 'B' constitute the 'shared pair'
♦ Both the Cl atoms have equal claim on both 'A' and 'B'
♦ So the two Cl atoms cannot move away from each other
■ 'Both the electrons in the pair' belongs to both the atoms
Feature 8: Using symbols
• It is not easy to draw 3D models of the cube for every atoms
• So Lewis developed a simplified method
• Only the electrons in the outer shell will take part in chemical reactions
• The electrons in the inner shells are well protected. In most cases, they do not take part in chemical reactions
■ Lewis noticed that, we need to show the outermost electrons only
♦ There electrons are shown as dots
♦ The dots are marked around the ‘symbol of atoms’
■ This notation is called Lewis symbol
• The fig.4.6 below shows the Lewis symbols of elements of the 2nd period
 |
| Fig.4.6 |
• The ‘number of dots’ in the Lewis symbol can be used to calculate the common valence or group valence
• When the number of dots is less than or equal to 4:
♦ Common valence = Number of dots
• When the number of dots is greater than 4:
♦ Common valence = 8 – number of dots
• The above given are the nine main features of the Lewis-Kossel model
(A postulate is 'something which is assumed to be true'. So that, it can be used as a basis for reasoning or discussion. The dictionary meaning can be seen here)
• They can be written in 6 steps
1. We know that:
• Alkali metals (group 1) are highly electropositive
• Halogens (group 17) are highly electronegative
2. Also we know that, in the periodic table,
• Alkali metals are at the left end
• Halogens are near the right end
3. Alkali metals being electropositive, readily lose their outermost single electron
• When that electron is lost, the atom becomes a +ve ion
♦ The +ve ion thus formed will be having the electronic configuration of a noble gas
♦ ‘Electronic configuration of a noble gas’ is a very stable configuration
♦ That means, the +ve ion formed from the ‘alkali metal atom’ will be very stable
4. Halogens being electronegative, readily accepts one more electron
• When that electron is gained, the atom becomes an -ve ion
♦ The -ve ion thus formed will be having the electronic configuration of a noble gas
♦ ‘Electronic configuration of a noble gas’ is a very stable configuration
♦ That means, the -ve ion formed from the ‘halogen atom’ will be very stable
5. So we have a 'stable +ve ion' and a 'stable -ve ion'
• An electrostatic force of attraction comes into play between the two oppositely charged ions
• Due to this electrostatic force of attraction, we will not be able to separate the two ions
♦ The two ions will always stick together
♦ Thus a molecule is formed
• We will see two examples:
Example 1: Formation of sodium chloride (NaCl)
• Na is an alkali metal
• It loses one electron as shown below:
$\mathbf\small{\rm{Na\longrightarrow Na^{+}+e^{-}}}$
♦ This is same as [Ne]3s1 becoming [Ne]
• Cl is a halogen
• It gains one electron as shown below:
$\mathbf\small{\rm{Cl+e^{-}\longrightarrow Cl^{-}}}$
♦ This is same as [Ne]3s23p5 becoming [Ne]3s23p6 or [Ar]
■ The Na+ and Cl- thus formed will stick together (due to electrostatic force of attraction) as shown below:
$\mathbf\small{\rm{Na^{+}+Cl^{-}\longrightarrow NaCl\,\,\;OR\;\,\,Na^{+}Cl^{-}}}$
• The fig.4.7 below shows the above result using Lewis symbols:
 |
| Fig.4.7 |
• Ca is an alkaline earth metal (group 2)
• It loses two electrons as shown below:
$\mathbf\small{\rm{Ca\longrightarrow Ca^{2+}+2e^{-}}}$
♦ This is same as [Ar]4s2 becoming [Ar]
• F is a halogen
• It gains one electron as shown below:
$\mathbf\small{\rm{F+e^{-}\longrightarrow F^{-}}}$
♦ This is same as [He]2s22p5 becoming [He]2s22p6 or [Ne]
■ The Ca+ and 2F- thus formed will stick together (due to electrostatic force of attraction) as shown below:
$\mathbf\small{\rm{Ca^{2+}+2F^{-}\longrightarrow CaF_2\,\,\;OR\;\,\,Ca^{2+}(F^{-})_2}}$
• The fig.4.8 below shows the above result using Lewis symbols:
 |
| Fig.4.8 |
♦ One Ca atom loses two electrons
♦ But one F atom can accept only one electron
♦ So 'two F atoms' will be required to accept 'the two electrons' lost by 'the one Ca'
6. In the above two examples, we see a 'chemical bonding' between two ions
• This chemical bonding helps in the formation of a molecule
• This chemical bonding is possible because of the electrostatic force of attraction between +ve and -ve ions
■ So Kossel called it: electrovalent bond
■ We can write the definition in one sentence:
The bond formed, as a result of the electrostatic attraction between the positive and negative ions was termed (by Kossel) as electrovalent bond
■ Kossel gave the definition for electrovalence also:
The charge possessed by an ion, when that ion is part of an electrovalent bond is called electrovalence
Some examples:
♦ Electrovalence of Na is +1
♦ Electrovalence of Ca is +2
♦ Electrovalence of Cl is -1
♦ Electrovalence of F is -1
■ Kossel's Postulates provided a strong foundation for further studies about 'structure of ionic compounds'. However, Kossel and other scientists of that time knew that, the 'structures of a large number of compounds' cannot be explained using these postulates. We will see them in later sections
Now we will see a solved example
Solved example 4.1
Mg. Na, B, O, N, Br
Solution:
The required Lewis dot symbols are shown in fig. below:
Sample explanation:
• Consider Br. It has the electronic configuration: 1s22s22p23s23p63d104s24p5 OR [Ar]3d104s24p5
• So the outermost main-shell has 7 electrons. That means, there are 7 valence electrons
• Thus there will be 7 dots in the Lewis dot symbol of Br
• In the next section, we will see the Octet rule. We will also see covalent bonds and Lewis dot structures

No comments:
Post a Comment