We want to learn about the Bohr model of atom. For that, we must first learn about 'wave nature' and 'particle nature' of electromagnetic radiations. In this regard,
• In section 2.5, we saw the 'wave nature of the electromagnetic radiations' and the 'electromagnetic spectrum'
• In section 2.6, we saw how 'particle nature' came to be known to the scientific community
• In the previous section 2.7, we saw how Albert Einstein proved the 'particle nature'. We also saw that the 'concept of dual nature' was accepted by the scientific community
• We are now one step closer to the Bohr model. The next step is to learn about 'quantization of energy possessed by electrons'. For that, we will first see 'atomic spectra' in this section
■ We have to learn about three types of spectra (‘Spectra’ is the plural of ‘spectrum’)
They are:
(a) The continuous spectrum
(b) The emission spectrum
(c) The absorption spectrum
• Similar bending occur for other colors also
2. But the ‘amount of bending’ is different for different colors
• Rays with shorter wavelengths bend more
• Rays with longer wavelengths bend less
3. So while passing through a prism, the violet bends the most while red bends the least
• Each of the other five colors suffer proportionate bending
4. Thus the seven component colors of the white light get separated from each other
• We have seen those basic details in our earlier classes (Details here)
■ The seven colors (seen separate from each other) constitute the visible spectrum
5. But there is an important point to note:
• There are no clear 'boundary lines' between the colors
• This is shown in fig.2.20 (a) below:
• In fig.a, we see that:
♦ red merges into orange
♦ orange merges into yellow
♦ yellow merges into green
♦ so on . . .
■ Since there are no boundaries in between the colors, it is called a continuous spectrum
1. Now consider fig.2.20(b)
• A sample of a gas at a low temperature is placed in between the light source and the prism
• The light has to pass through the gas before reaching the prism
2. So whatever light reaching the prism has passed through the gas
• While passing through the gas, does the light suffer any change?
• To answer that question, we look at the resulting spectrum
• While passing through the prism, the components of the light get separated
• The resulting spectrum is shown in the fig.b
3. We see some black lines in the resulting spectrum
• 'Black lines' because they have no color
• Those black lines are gaps
4. Gaps are formed because, the colors corresponding to those lines are missing
• Light waves of those missing colors were absorbed by the gas
5. Since there are gaps, it is not a continuous spectrum
• It is a discontinuous spectrum
■ We can write:
♦ This spectrum is produced by a light
♦ Some of the components of that light were absorbed
♦ So this spectrum is called an absorption spectrum
1. Now consider fig.2.20(c)
• Another sample of the same gas is now at a high temperature
2. There is no 'external light source'
• The only light is the 'light emitted from the hot gas'
3. Does this 'emitted light' have any specialty ?
• To answer that question, we look at the resulting spectrum
• While passing through the prism, the components of the light get separated
• The resulting spectrum is shown in the fig.c
4. We see that, there are only certain lines
• There are large gaps in between the lines
• So this is also not a continuous spectrum
5. Consider the left most line
• It is red in color
• We know that the 'wave length of red' varies from 750 to 610 nm
• A particular wavelength between 750 and 610 was emitted by the hot gas
• Only a ‘single value wavelength’ was emitted in the red region
♦ That is why, we get only a ‘red line’
• A ‘range of red wavelengths’ was not emitted. Only a ‘single value wavelength’ was emitted
♦ That is why, we do not get a ‘red region’
• Interestingly, this same red line is missing from the absorption spectrum in fig.b
6. Consider the second left most line
• It is orange in color
• We know that the 'wave length of orange' varies from 610 to 590 nm
• A particular wavelength between 610 and 590 was emitted by the hot gas
• Only a ‘single value wavelength’ was emitted in the orange region
♦ That is why, we get only a ‘orange line’
• A ‘range of orange wavelengths’ was not emitted. Only a ‘single value wavelength’ was emitted
♦ That is why, we do not get a ‘orange region’
• Interestingly, this same orange line is missing from the absorption spectrum in fig.b
7. The steps like (5) and (6) can be written for the other lines in fig.c also
• The other lines are yellow, green, green and blue in color
8. Note that, the lines that appear in the emission spectrum, are missing in the absorption spectrum
So we can write:
The absorption spectrum is like the photographic negative of the emission spectrum
9. The emission spectrum in fig.c, has 'lines' only
Such spectra are called line spectra
1. The study of emission or absorption spectra is called spectroscopy
2. The emission spectra (also called line spectra) is unique for each element
• That means:
♦ Each element has it’s own line spectra
♦ It is just like each individual having his/her own finger prints
3. Suppose that, an unknown element is brought to the lab for identification
(i) A small sample of that element is heated
(ii) The radiations emitted from the sample is passed through a prism
• The components of the radiations get separated from each other
(iii) The separated components are allowed to fall on a photographic plate
• Thus we get the emission spectrum
(iv) This emission spectrum is compared with the emission spectrum of known elements
• Thus the unknown element can be identified
(v) Instead of heating, electricity can be passed through the element to obtain the spectrum
4. Many elements like Rubedium, Caesium etc., were discovered when their minerals were analyzed using spectroscopic methods
• The presence of helium in the sun was also discovered when the light coming from the sun was analyzed using spectroscopic methods
■ Our next aim is to find ‘how those distinct lines are formed’
A simplified explanation can be given in eight steps as follows:
1. In fig.2.21 (a) below, an electron is moving around the nucleus
• The electron cannot take ‘any path’ around the nucleus
• There are certain ‘fixed paths’. These fixed paths are called orbitals
2. In fig.b, white light is falling on the atom
• When white light falls on the sample, the electron absorbs energy
3. After the absorption, the electron will have a ‘higher energy content’
So it moves to a higher orbital. This is shown in fig.c
4. Before absorption of energy, the electron is said to be in the ground state
• So in the fig.2.20 (b) that we saw previously, the electrons in the cold gas are initially in the ground state
5. When light (or electricity) is applied, those electrons absorb energy
• An electron which has absorbed energy is said to be in the excited state
• So in that fig.2.20(c), the electrons in the hot gas are in the excited state
6. So what happens when the electrons reach the excited state?
Answer:
• They cannot remain in the excited state for long
• They will try to return to the ground state
• For that, they give off the ‘energy that was absorbed’
• They give off the energy and jumps back to the ground state
• This is shown in fig.2.22 below:
• In fig.2.22 (a), the electron is in the excited state. It gives off energy
• In fig.b, the electron is shown back at the ground state
7. In step (2), we saw that ‘energy absorption’ takes place
• That means, some of the waves are absorbed from the white light
• Those absorbed waves will not come out of the atom
• So those absorbed waves will be seen as black lines in the resulting absorption spectrum
8. In step (6), we saw that ‘energy emission’ takes place
• The ‘amount of energy emitted’ is exactly equal to the ‘amount of energy absorbed’
• That means, the emitted wave will be of the same color as the absorbed wave
• So ‘the missing lines in the absorption spectrum’ are obtained back in the emission spectrum
• This is because, an electron will not absorb ‘any energy’.
• It does not matter whether the ‘energy available for absorption’ is high or low.
• But it must be ‘a suitable quantity of energy’
• The electron selects only ‘suitable quantities of energy’ and absorbs them
■ What is the criterion for that ‘suitability’?
We will see it in the next section in which we take 'the electron in hydrogen' as an example
• In section 2.5, we saw the 'wave nature of the electromagnetic radiations' and the 'electromagnetic spectrum'
• In section 2.6, we saw how 'particle nature' came to be known to the scientific community
• In the previous section 2.7, we saw how Albert Einstein proved the 'particle nature'. We also saw that the 'concept of dual nature' was accepted by the scientific community
• We are now one step closer to the Bohr model. The next step is to learn about 'quantization of energy possessed by electrons'. For that, we will first see 'atomic spectra' in this section
■ We have to learn about three types of spectra (‘Spectra’ is the plural of ‘spectrum’)
They are:
(a) The continuous spectrum
(b) The emission spectrum
(c) The absorption spectrum
Continuous spectrum
1. Consider a ray of red light. When it passes from one medium to another, say from air to glass, 'bending of the ray' occurs• Similar bending occur for other colors also
2. But the ‘amount of bending’ is different for different colors
• Rays with shorter wavelengths bend more
• Rays with longer wavelengths bend less
3. So while passing through a prism, the violet bends the most while red bends the least
• Each of the other five colors suffer proportionate bending
4. Thus the seven component colors of the white light get separated from each other
• We have seen those basic details in our earlier classes (Details here)
■ The seven colors (seen separate from each other) constitute the visible spectrum
5. But there is an important point to note:
• There are no clear 'boundary lines' between the colors
• This is shown in fig.2.20 (a) below:
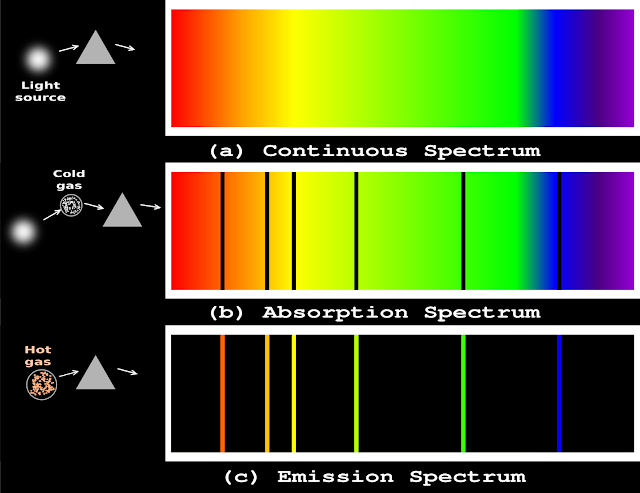 |
| Fig.2.20 |
♦ red merges into orange
♦ orange merges into yellow
♦ yellow merges into green
♦ so on . . .
■ Since there are no boundaries in between the colors, it is called a continuous spectrum
Absorption spectrum
1. Now consider fig.2.20(b)
• A sample of a gas at a low temperature is placed in between the light source and the prism
• The light has to pass through the gas before reaching the prism
2. So whatever light reaching the prism has passed through the gas
• While passing through the gas, does the light suffer any change?
• To answer that question, we look at the resulting spectrum
• While passing through the prism, the components of the light get separated
• The resulting spectrum is shown in the fig.b
3. We see some black lines in the resulting spectrum
• 'Black lines' because they have no color
• Those black lines are gaps
4. Gaps are formed because, the colors corresponding to those lines are missing
• Light waves of those missing colors were absorbed by the gas
5. Since there are gaps, it is not a continuous spectrum
• It is a discontinuous spectrum
■ We can write:
♦ This spectrum is produced by a light
♦ Some of the components of that light were absorbed
♦ So this spectrum is called an absorption spectrum
Emission spectrum
1. Now consider fig.2.20(c)
• Another sample of the same gas is now at a high temperature
2. There is no 'external light source'
• The only light is the 'light emitted from the hot gas'
3. Does this 'emitted light' have any specialty ?
• To answer that question, we look at the resulting spectrum
• While passing through the prism, the components of the light get separated
• The resulting spectrum is shown in the fig.c
4. We see that, there are only certain lines
• There are large gaps in between the lines
• So this is also not a continuous spectrum
5. Consider the left most line
• It is red in color
• We know that the 'wave length of red' varies from 750 to 610 nm
• A particular wavelength between 750 and 610 was emitted by the hot gas
• Only a ‘single value wavelength’ was emitted in the red region
♦ That is why, we get only a ‘red line’
• A ‘range of red wavelengths’ was not emitted. Only a ‘single value wavelength’ was emitted
♦ That is why, we do not get a ‘red region’
• Interestingly, this same red line is missing from the absorption spectrum in fig.b
6. Consider the second left most line
• It is orange in color
• We know that the 'wave length of orange' varies from 610 to 590 nm
• A particular wavelength between 610 and 590 was emitted by the hot gas
• Only a ‘single value wavelength’ was emitted in the orange region
♦ That is why, we get only a ‘orange line’
• A ‘range of orange wavelengths’ was not emitted. Only a ‘single value wavelength’ was emitted
♦ That is why, we do not get a ‘orange region’
• Interestingly, this same orange line is missing from the absorption spectrum in fig.b
7. The steps like (5) and (6) can be written for the other lines in fig.c also
• The other lines are yellow, green, green and blue in color
8. Note that, the lines that appear in the emission spectrum, are missing in the absorption spectrum
So we can write:
The absorption spectrum is like the photographic negative of the emission spectrum
9. The emission spectrum in fig.c, has 'lines' only
Such spectra are called line spectra
Spectroscopy
1. The study of emission or absorption spectra is called spectroscopy
2. The emission spectra (also called line spectra) is unique for each element
• That means:
♦ Each element has it’s own line spectra
♦ It is just like each individual having his/her own finger prints
3. Suppose that, an unknown element is brought to the lab for identification
(i) A small sample of that element is heated
(ii) The radiations emitted from the sample is passed through a prism
• The components of the radiations get separated from each other
(iii) The separated components are allowed to fall on a photographic plate
• Thus we get the emission spectrum
(iv) This emission spectrum is compared with the emission spectrum of known elements
• Thus the unknown element can be identified
(v) Instead of heating, electricity can be passed through the element to obtain the spectrum
4. Many elements like Rubedium, Caesium etc., were discovered when their minerals were analyzed using spectroscopic methods
• The presence of helium in the sun was also discovered when the light coming from the sun was analyzed using spectroscopic methods
• So now we know how the emission spectra and absorption spectra are obtained
A simplified explanation can be given in eight steps as follows:
1. In fig.2.21 (a) below, an electron is moving around the nucleus
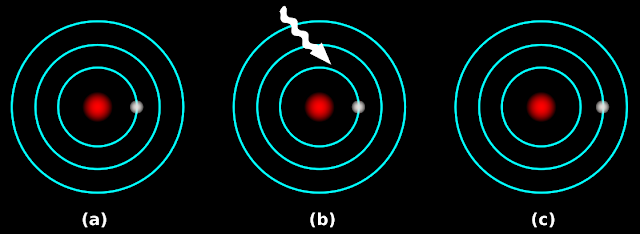 |
| Fig.2.21 |
• There are certain ‘fixed paths’. These fixed paths are called orbitals
2. In fig.b, white light is falling on the atom
• When white light falls on the sample, the electron absorbs energy
3. After the absorption, the electron will have a ‘higher energy content’
So it moves to a higher orbital. This is shown in fig.c
4. Before absorption of energy, the electron is said to be in the ground state
• So in the fig.2.20 (b) that we saw previously, the electrons in the cold gas are initially in the ground state
5. When light (or electricity) is applied, those electrons absorb energy
• An electron which has absorbed energy is said to be in the excited state
• So in that fig.2.20(c), the electrons in the hot gas are in the excited state
6. So what happens when the electrons reach the excited state?
Answer:
• They cannot remain in the excited state for long
• They will try to return to the ground state
• For that, they give off the ‘energy that was absorbed’
• They give off the energy and jumps back to the ground state
• This is shown in fig.2.22 below:
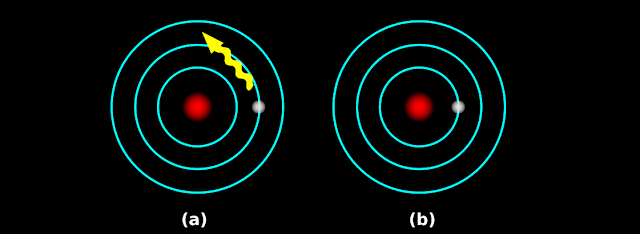 |
| Fig.2.22 |
• In fig.b, the electron is shown back at the ground state
7. In step (2), we saw that ‘energy absorption’ takes place
• That means, some of the waves are absorbed from the white light
• Those absorbed waves will not come out of the atom
• So those absorbed waves will be seen as black lines in the resulting absorption spectrum
8. In step (6), we saw that ‘energy emission’ takes place
• The ‘amount of energy emitted’ is exactly equal to the ‘amount of energy absorbed’
• That means, the emitted wave will be of the same color as the absorbed wave
• So ‘the missing lines in the absorption spectrum’ are obtained back in the emission spectrum
• The above eight steps give a simplified explanation for the lines. But things are not that simple
• It does not matter whether the ‘energy available for absorption’ is high or low.
• But it must be ‘a suitable quantity of energy’
• The electron selects only ‘suitable quantities of energy’ and absorbs them
■ What is the criterion for that ‘suitability’?
We will see it in the next section in which we take 'the electron in hydrogen' as an example
No comments:
Post a Comment