In the previous section, we saw the details about homolytic cleavage. In this section, we will see nucleophiles and electrophiles.
Details about nucleophiles can be written in 7 steps:
1. Consider the species shown in fig.12.80(a) below.
 |
| Fig.12.80 |
• Both O and H atoms have octet.
• But the blue dot of O originally belonged to some other atom. Recall that, O does not have seven valence electrons. It has only six.
• So the blue dot gives a -ve charge to the species.
• The species also has lone pairs of electrons.
• Such species will be always seeking nuclei of other atoms. We know that nuclei represent +ve charges because of the presence of protons.
◼ So the species with negative charge and lone pairs will be seeking nuclei so that they can donate electrons to those nuclei. Such species are called nucleophiles. They are represented as Nu:
2. Fig.12.80(b) shows another Nu:
• It is the cyanide ion (CN-).
• From the Lewis structure, we can see that, both C and N atoms have octet.
• But the red dot of C originally belonged to some other atom. Recall that, C does not have five valence electrons. It has only four.
• So the red dot gives a -ve charge to the species.
• The species also has lone pairs of electrons.
3. Fig.c shows a carbanion that we saw in a previous section. See fig.12.73 of section 12.10. We have seen how it attained the -ve charge.
• Fig.c shows the methyl carbanion. We represent it as: $\mathbf{\rm{{H_3}\overset{-}{C}}}$
• There can be ethyl carbanion also: $\mathbf{\rm{CH_3\,{H_2}\overset{-}{C}}}$
• There can be propyl carbanion also: $\mathbf{\rm{CH_3\,CH_3\,{H_2}\overset{-}{C}}}$
• Such carbanions can be represented in general as: $\mathbf{\rm{R{H_3}\overset{-}{C}}}$
Carbanions also come under the category of Nu:
4. Fig.12.81(a) below shows the Lewis structure of water.
 |
| Fig.12.81 |
• We see that, both the H atoms and the O atom have octet.
• O has six valence electrons. But four of them are not participating in bonds. So there are two lone pairs. Presence of lone pairs makes water a Nu:
5. Fig.12.81(b) above shows the Lewis structure of (CH3)3N.
• We see that, all atoms have octet.
•
N has five valence electrons. But two of them are not participating in
bonds. So there is a lone pair. Presence of lone pairs makes (CH3)3N a
Nu:
• In the place of CH3, we can have any alkyl group. The resulting structure will give N a lone pair.
• So we can write: R3N is a
Nu:
6. Fig.12.81(b) above shows the Lewis structure of CH3NHCH3.
• We see that, all atoms have octet.
•
N has five valence electrons. But two of them are not participating in
bonds. So there is a lone pair. Presence of lone pairs makes CH3NHCH3 a
Nu:
• In the place of CH3, we can have any alkyl group. The resulting structure will give N a lone pair.
• So we can write: R2NH is a
Nu:
7. The above six examples help us to get a basic understanding about nucleophiles. We can say that, nucleophiles are ready to donate electrons.
Next we will learn about electrophiles. It can be written in 4 steps:
1. Consider the carbocation that we saw in a previous section. See fig.12.68 of section 12.10.
• We have seen how it attained the +ve charge.
• Because of the +ve charge, these species always seek electrons. So they can be called electrophiles. They are represented as E+.
2. Even neutral molecules (molecules with no charge) can act as electrophiles. Alkyl halide is an example. Let us see how an alkyl halide can act as an electrophile. It can be written in 4 steps:
(i) Consider the structure of CH3Br in fig.12.82(a) below:
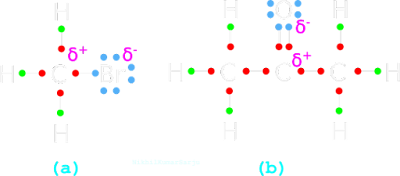 |
| Fig.12.82 |
(ii) One of the bonds is between C and Br.
• The Br has a high electronegativity. So both the electrons in the bond will be attracted towards the Br.
• Thus the C atom will get a small +ve charge (δ+).
(iii) Now, if a nucleophile is any where in the vicinity of the CH3Br, it will attack the C atom with the δ+ charge. So clearly, CH3Br acts as a E+.
(iv) Alkyl halides are in general, E+.
• This is because, the halogens F, Cl, Br and I can pull electrons away from the C atom.
• Alkyl halides can be represented in the general form as: R3CㅡX, where X is a halogen atom.
• So we can write: R3ㅡX are electrophiles.
3. Ketones (hydrocarbons with carbonyl group) is another example for neutral molecules which act as
E+. It can be written in 4 steps:
(i) Consider the structure of CH3ㅡCOㅡCH3 (propanone) in fig.12.82(b) above.
(ii) One of the bonds is between C and O. It is a double bond
• The O has a high electronegativity. So all four electrons in the bond will be attracted towards the O.
♦ Since it is a double bond, there are four electrons.
♦ Pulling of four electrons will give a greater δ+ for the C atom.
♦ So the double bond creates greater δ+.
(iii) Now, if a nucleophile is any where in the vicinity of the CH3ㅡCOㅡCH3, it will attack the C atom with the δ+ charge. So clearly, CH3ㅡCOㅡCH3 acts as a E+.
(iv) Ketones are in general, E+.
• This is because of the pull by O atom at the double bond in the carbonyl group.
4. The above examples help us to get a basic understanding about
electrophiles. We can say that, electrophiles are
ready to accept electrons.
Now we will see some solved examples:
Solved example 12.14
Giving justification, categorize the following molecules/ions as nucleophile or electrophile.
(a) HS- (b) BF3 (c) C2H5O- (d) (CH3)3N: (e) $\mathbf{\rm{\overset{+}{Cl}}}$ (f) $\mathbf{\rm{CH_3\,\overset{+}{C}=O}}$ (g) H2N:- (h) $\mathbf{\rm{\overset{+}{N} O_2}}$
Solution:
Part (a): HS-
• Fig.12.83(a) below shows the Lewis structure of HS-
 |
| Fig.12.83 |
• But the blue dot of S originally belonged to some other atom. Recall that, S does not have seven valence electrons. It has only six.
• So the blue dot gives a -ve charge to the species.
• The species also has lone pairs of electrons.
• So it is a Nu:
Part (b): BF3
• Fig.12.83(b) shows the Lewis structure of BF3
• F atoms have octet. But the B atom has only sextet.
• Since B atom needs two more electrons, the species as a whole, is a E+
Part (c): C2H5O-
• Fig.12.83(c) above shows the Lewis structure of C2H5O-
• All the C, H and O atoms have octet.
• But the blue dot of O originally belonged to some other atom. Recall that, O does not have seven valence electrons. It has only six.
• So the blue dot gives a -ve charge to the species.
• The species also has lone pairs of electrons.
• So it is a Nu:
Part (d): (CH3)3N: C2H5O-
• We have already seen this species. It is a Nu:
• See fig.12.81(b) at the beginning of this section.
Part (e): $\mathbf{\rm{\overset{+}{Cl}}}$
• We know that, Cl originally has 7 valence electrons.
• It usually gets one more electron from a suitable source and thus attains octet (Cl-).
• But in our present case, Cl has lost one of it's original 7 valence electrons. Thus it became $\mathbf{\rm{\overset{+}{Cl}}}$
• So it is electron deficient and hence a E+
• The Lewis structure is shown in fig.12.84(a) below:
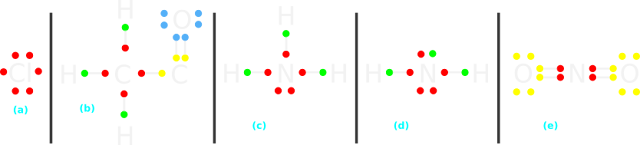 |
| Fig.12.84 |
• Fig.12.84(b) above shows the Lewis structure.
• Originally, there was a bond on the right side of the C atom. During heterolysis, that bond was cleaved in such a way that both the electrons in that bond left the C atom.
• So the species needs electrons. It is a E+
Part (g): H2N:-
• Fig.12.84(c) above shows the Lewis structure of NH3.
• From NH3, an H atom leaves after giving it's electron to N. This is the green dot above N in fig.d
• The N atom originally has five valence electrons. The five red dots. Now it has the extra green dot. So the species has a negative charge. It is a Nu:
Part (h): $\mathbf{\rm{\overset{+}{N} O_2}}$
• Fig.12.84(e) above shows the Lewis structure of $\mathbf{\rm{\overset{+}{N} O_2}}$
• Both O and N atoms have octet.
• But we see that, one red dot of N is missing. There should have been five red dots, indicating the five valence electrons of N.
• So N has lost an electron, giving it a +ve charge.
• Thus the species as a whole is an E+
Solved example 12.15
Identify electrophilic center in the following:
(a) CH3CH=O (b) CH3CN (c) CH3I
Solution:
Part (a): CH3CH=O
• Fig.12.85(a) below shows the Lewis structure of CH3CH=O
 |
| Fig.12.85 |
• The O atom, being more electronegative than C, pulls the electrons. This effect is even more enhanced due to the double bond. This is because, the O atom will pull four electrons present in the
double bond, thus giving a greater positive charge for the C atom.
• Thus the C atom gets a partial positive charge δ+. It becomes an electrophilic center.
Part (b): CH3CH=O
• Fig.12.85(b) above shows the Lewis structure of CH3CH=O
• The N atom, being more electronegative than C, pulls the electrons. This effect is even more enhanced due to the triple bond. This is because, the N atom will pull six electrons present in the triple bond, thus giving a greater positive charge for the C atom.
• Thus the C atom gets a partial positive charge δ+. It becomes an electrophilic center.
Part (c): CH3I
• Fig.12.85(c) above shows the Lewis structure of CH3I
•
The I atom, being more electronegative than C, pulls the electrons.
• Thus the C atom gets a partial positive charge δ+. It becomes an electrophilic center.
Now we know the basics about nucleophiles and electrophiles. It is clear that, they can help each other during a chemical reaction. This is because, the electrophiles are looking for electrons and those electrons can be supplied by nucleophiles.
In the next section, we
will see electron movement in organic reactions.
Previous
Contents
Next
Copyright©2021 Higher secondary chemistry.blogspot.com
No comments:
Post a Comment