In the previous section, we saw the dipole moments in HF, H2O, BeF2 and BF3. In this section, we will see the dipole moments in NH3 and NF3
First we have to learn about the structure of a NH3 molecule. It can be done in 5 steps:
1. Consider a pyramid
• The pyramid that we have seen in our earlier math classes had a square base. It was called a square pyramid
♦ So it had 4 lateral faces. All those 4 faces were triangles
• In our present case, the base of the pyramid is a triangle. It is called a triangular pyramid
♦ So it has 3 lateral faces. All those 3 faces are triangles
♦ So altogether, our present pyramid has 4 triangular faces
2. There is an important speciality about the triangular pyramids that we consider here. It can be written in 2 steps:
(i) All the 4 triangles are identical (congruent)
(ii) All the 4 triangles are equilateral triangles
♦ An equilateral triangle has all the three sides equal
♦ Also all the three angles are 60o
• Such a triangular pyramid is shown in fig.4.70(a) below:
• In fig.a, we see only two lateral faces. The third lateral face and the base are not visible
• If we remove the yellow face and give some transparency to the orange face, the other faces will also become visible. This is shown in fig.b
♦ In fig.b, we can see the base triangle (white color) and the opposite triangle (magenta color)
3. The NH3 molecule has the shape of a triangular pyramid
• This can be explained in 4 steps:
(i) The NH3 molecule has one N atom and three H atoms
(ii) The N atom occupies the apex of the pyramid
(iii) The H atoms occupy the three corners of the base
(iv) The covalent bonds act along the lateral edges of the pyramid
• This is shown in fig.4.71(a) below:
4. From the fig.4.71(a), the orientations of various atoms in the NH3 molecule are clear
• This is a ball-and-stick model
♦ The balls represent the atoms
✰ We see that, the balls occupy the corners of the pyramid
♦ The sticks represent the bonds
✰ We see that, the sticks are oriented along the lateral edges of the pyramid
• Now we can remove the faces of the pyramid
• The result is shown in fig.4.71(b) above
5. But the structure is not yet complete
• In the Lewis dot structure of NH3, we see a 'lone pair of electrons'
• This lone pair must also be shown in the 3D model
• So the final structure will be as shown in the fig.4.72 below:
• We will see the 'reason for this pyramidal shape' in later sections. At present, we are more concerned about dipole moments
5. Fig.4.72 represents the shape of an NH3 molecule
• It is a 3D shape. We will need to represent the shape of NH3 many times in our note books and records. It is not easy to draw such 3D shapes every time. So we adopt another method. It can be explained in 4 steps:
(i) Points and planes
• Consider any one point in space
♦ We can easily draw a plane passing through that point
✰ In fact, there will be infinite number of planes passing through that point
• Consider any two points in space
♦ We can easily draw a plane passing through both those points
✰ In fact, there will be infinite number of planes passing through both those points
• Consider any three points in space
♦ We can draw a plane passing through all those three points (Details here)
✰ But, there will be only one plane passing through all those three points
• Consider any four points in space
♦ We can draw a plane passing through any three of those four points
✰ There is no guarantee that, the fourth point also lies on that point
(ii) So we see that 'three points' is the maximum possible for a guaranteed plane
• Let us take 3 atoms from our NH3 molecule
♦ Our selection must include the central atom, which is N
• So we take the N and any two H atoms
• We can draw a plane through the 3 atoms. This is shown in fig.4.73(a) below:
■ This plane is the 'plane of our paper' or the 'plane of the computer screen'
(iii) Atoms in the front and rear of plane
• One H atom falls in front of the plane
♦ Atoms in front of the plane are represented using a solid wedge as shown in fig.b
• In our present case, there are no atoms behind the plane
♦ Atoms behind the plane are represented using a dashed wedge as shown in fig.c
• The N and two H atoms fall exactly on the plane
♦ Such atoms are connected by simple solid lines that we commonly use in Lewis structures
(iv) The final structure is shown in fig.d
• Such representations are called wedge-dash-line models
1. In this case, there are three bonds
♦ All of them are N-H bonds
♦ Those bonds do not lie on the same line
2. The orientations of the bonds can be described in two steps:
(i) The central atom N is situated at the apex of the triangular pyramid
(ii) The 3 bonds start from the N atom
♦ They are directed towards the corners of the 'base of the pyramid'
3. From the 3 bonds, we will have 3 dipole moments
• They are shown in yellow color in fig.4.74(a) below:
• The dipole moments are due to the 'pulling of electrons' by the N atom
4. In addition to the above 3 dipole moments, there is one more. It can be explained in 4 steps:
(i) Consider the 'lone pair electrons' of N atom
• This pair is at a certain distance, away from the nucleus of the N atom
(ii) The nucleus of the N atom is positively charged
• The electrons are negatively charged
(iii) So there are two opposite charges
• Also, there is a distance between the two 'opposite charges'
• Thus a dipole moment comes into effect
(iv) This is called the 'dipole moment due to lone pairs'
• It is shown in orange color in the fig.4.74(a) above
5. So there are a total of 4 dipole moments
• But we want the dipole moment of the ‘molecule as a whole’
■ For that, we calculate the vector sum
• We have seen that, dipole moments are vector quantities
♦ So they can be added using 'principles of vector addition'
✰ Details can be seen here
6. We do the addition in two steps
• In step 1, we add the yellow vectors and find their resultant
• In step 2, we add the 'resultant obtained in step 1' with the orange vector
Step 1:
(i) To add the yellow vectors, we resolve them into rectangular components
• This is shown in fig.4.74(b) above
♦ The horizontal components are shown in red color
♦ The vertical components are shown in green color
(ii) The base triangle of the pyramid is an equilateral triangle
• So the horizontal reds meet symmetrically at the center of the triangle
• This is shown in fig.4.74(c) above
• They cancel out giving a null vector
(iii) The vertical greens add up
• The result is a thick vertical green vector shown in fig.4.75(a) below:
Step 2:
(i) In this step, we add the two vectors shown in fig.4.75(a)
• Obviously, the resultant will be a vertical vector pointing upwards
(ii) This resultant vector is shown in magenta color in fig.4.75(b) above
7. So the magenta vector in fig.4.75(b) indicates the resultant dipole moment in NH3
• Scientists have determined it's value as 4.90 × 10-30 C m
• This value can be converted into Debye units
♦ We have: 1 D = 3.33564 × 10-30 C m
♦ ⇒ 1 C m = [1 ÷ (3.33564 × 10-30)] D
♦ ⇒ 4.90 × 10-30 C m = (4.90 × 10-30 ) × [1 ÷ (3.33564 × 10-30)] = 1.47 D
• First we have to learn about the structure of a NF3 molecule. It is similar to that of NH3
• The only difference is that:
♦ In NH3, there is a H atom at each of the three corners of the base
♦ In NF3, there is a F atom at each of the three corners of the base
• So the final structure will be as shown in the fig.4.76(a) below. The wedge-dash-line model is shown in fig.b
We can start the discussion about it's dipole moment
1. In this case, there are three bonds
♦ All of them are N-F bonds
♦ Those bonds do not lie on the same line
2. The orientations of the bonds can be described in two steps:
(i) The central atom N is situated at the apex of the triangular pyramid
(ii) The 3 bonds start from the N atom
♦ They are directed towards the corners of the 'base of the pyramid'
3. From the 3 bonds, we will have 3 dipole moments
• They are shown in yellow color in fig.4.77(a) below:
• The dipole moments are due to the 'pulling of electrons' by the F atoms
• Recall that, in the NH3 molecule, the dipole moments are due to the 'pulling of electrons' by the N atom
• So the 3 dipole moments in NF3 are opposite in directions when compared to NH3
4. In addition to the above 3 dipole moments, there is one more
• It is the same 'dipole moment due to lone pairs' that we saw in the case of NH3
• It is shown in orange color in the fig.4.77(a) above
5. So there are a total of 4 dipole moments
• But we want the dipole moment of the ‘molecule as a whole’
■ For that, we calculate the vector sum
• We have seen that, dipole moments are vector quantities
♦ So they can be added using 'principles of vector addition'
✰ Details can be seen here
6. We do the addition in two steps
• In step 1, we add the yellow vectors and find the resultant
• In step 2, we add the 'resultant obtained in step 1' with the orange vector
Step 1:
(i) To add the yellow vectors, we resolve them into rectangular components
• This is shown in fig.4.77(b) above
♦ The horizontal components are shown in red color
♦ The vertical components are shown in green color
(ii) The base triangle of the pyramid is an equilateral triangle
• So the horizontal reds pull symmetrically away from the center of the triangle
• This is shown in fig.4.77(c) above
• They cancel out giving a null vector
(iii) The vertical greens add up
• The result is a thick vertical green vector shown in fig.4.78(a) below:
Step 2:
(i) In this step, we add the two vectors shown in fig.4.78(a)
• Obviously, the resultant will be a vertical vector
(ii) But it's direction should be carefully determined
• The direction can be 'vertically upwards' or 'vertically downwards'
(iii) In fig.4.78(a), we have two vectors:
• The green and the orange
♦ They act in opposite directions
♦ The green is larger in magnitude
• So the resultant of green and orange will be acting downwards
(ii) This resultant vector is shown in magenta color in fig.4.78(b) above
7. So the magenta vector in fig.4.78(b) indicates the resultant dipole moment in NF3
• Scientists have determined it's value as 0.80 × 10-30 C m
• This value can be converted into Debye units
♦ We have: 1 D = 3.33564 × 10-30 C m
♦ ⇒ 1 C m = [1 ÷ (3.33564 × 10-30)] D
♦ ⇒ 0.80 × 10-30 C m = (0.80 × 10-30 ) × [1 ÷ (3.33564 × 10-30)] = 0.23 D
Dipole moment in NH3
First we have to learn about the structure of a NH3 molecule. It can be done in 5 steps:
1. Consider a pyramid
• The pyramid that we have seen in our earlier math classes had a square base. It was called a square pyramid
♦ So it had 4 lateral faces. All those 4 faces were triangles
• In our present case, the base of the pyramid is a triangle. It is called a triangular pyramid
♦ So it has 3 lateral faces. All those 3 faces are triangles
♦ So altogether, our present pyramid has 4 triangular faces
2. There is an important speciality about the triangular pyramids that we consider here. It can be written in 2 steps:
(i) All the 4 triangles are identical (congruent)
(ii) All the 4 triangles are equilateral triangles
♦ An equilateral triangle has all the three sides equal
♦ Also all the three angles are 60o
• Such a triangular pyramid is shown in fig.4.70(a) below:
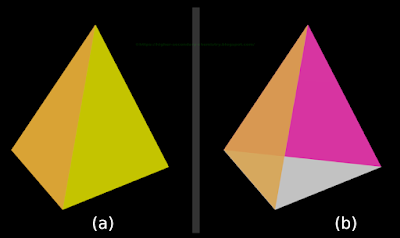 |
| Fig.4.70 |
• If we remove the yellow face and give some transparency to the orange face, the other faces will also become visible. This is shown in fig.b
♦ In fig.b, we can see the base triangle (white color) and the opposite triangle (magenta color)
3. The NH3 molecule has the shape of a triangular pyramid
• This can be explained in 4 steps:
(i) The NH3 molecule has one N atom and three H atoms
(ii) The N atom occupies the apex of the pyramid
(iii) The H atoms occupy the three corners of the base
(iv) The covalent bonds act along the lateral edges of the pyramid
• This is shown in fig.4.71(a) below:
 |
| Fig.4.71 |
• This is a ball-and-stick model
♦ The balls represent the atoms
✰ We see that, the balls occupy the corners of the pyramid
♦ The sticks represent the bonds
✰ We see that, the sticks are oriented along the lateral edges of the pyramid
• Now we can remove the faces of the pyramid
• The result is shown in fig.4.71(b) above
5. But the structure is not yet complete
• In the Lewis dot structure of NH3, we see a 'lone pair of electrons'
• This lone pair must also be shown in the 3D model
• So the final structure will be as shown in the fig.4.72 below:
 |
| Fig.4.72 |
5. Fig.4.72 represents the shape of an NH3 molecule
• It is a 3D shape. We will need to represent the shape of NH3 many times in our note books and records. It is not easy to draw such 3D shapes every time. So we adopt another method. It can be explained in 4 steps:
(i) Points and planes
• Consider any one point in space
♦ We can easily draw a plane passing through that point
✰ In fact, there will be infinite number of planes passing through that point
• Consider any two points in space
♦ We can easily draw a plane passing through both those points
✰ In fact, there will be infinite number of planes passing through both those points
• Consider any three points in space
♦ We can draw a plane passing through all those three points (Details here)
✰ But, there will be only one plane passing through all those three points
• Consider any four points in space
♦ We can draw a plane passing through any three of those four points
✰ There is no guarantee that, the fourth point also lies on that point
(ii) So we see that 'three points' is the maximum possible for a guaranteed plane
• Let us take 3 atoms from our NH3 molecule
♦ Our selection must include the central atom, which is N
• So we take the N and any two H atoms
• We can draw a plane through the 3 atoms. This is shown in fig.4.73(a) below:
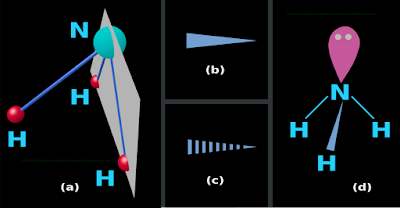 |
| Fig.4.73 |
(iii) Atoms in the front and rear of plane
• One H atom falls in front of the plane
♦ Atoms in front of the plane are represented using a solid wedge as shown in fig.b
• In our present case, there are no atoms behind the plane
♦ Atoms behind the plane are represented using a dashed wedge as shown in fig.c
• The N and two H atoms fall exactly on the plane
♦ Such atoms are connected by simple solid lines that we commonly use in Lewis structures
(iv) The final structure is shown in fig.d
• Such representations are called wedge-dash-line models
So now we have an idea about the structure of the NH3 molecule. We can start the discussion about it's dipole moment
♦ All of them are N-H bonds
♦ Those bonds do not lie on the same line
2. The orientations of the bonds can be described in two steps:
(i) The central atom N is situated at the apex of the triangular pyramid
(ii) The 3 bonds start from the N atom
♦ They are directed towards the corners of the 'base of the pyramid'
3. From the 3 bonds, we will have 3 dipole moments
• They are shown in yellow color in fig.4.74(a) below:
 |
| Fig.4.74 |
4. In addition to the above 3 dipole moments, there is one more. It can be explained in 4 steps:
(i) Consider the 'lone pair electrons' of N atom
• This pair is at a certain distance, away from the nucleus of the N atom
(ii) The nucleus of the N atom is positively charged
• The electrons are negatively charged
(iii) So there are two opposite charges
• Also, there is a distance between the two 'opposite charges'
• Thus a dipole moment comes into effect
(iv) This is called the 'dipole moment due to lone pairs'
• It is shown in orange color in the fig.4.74(a) above
5. So there are a total of 4 dipole moments
• But we want the dipole moment of the ‘molecule as a whole’
■ For that, we calculate the vector sum
• We have seen that, dipole moments are vector quantities
♦ So they can be added using 'principles of vector addition'
✰ Details can be seen here
6. We do the addition in two steps
• In step 1, we add the yellow vectors and find their resultant
• In step 2, we add the 'resultant obtained in step 1' with the orange vector
Step 1:
(i) To add the yellow vectors, we resolve them into rectangular components
• This is shown in fig.4.74(b) above
♦ The horizontal components are shown in red color
♦ The vertical components are shown in green color
(ii) The base triangle of the pyramid is an equilateral triangle
• So the horizontal reds meet symmetrically at the center of the triangle
• This is shown in fig.4.74(c) above
• They cancel out giving a null vector
(iii) The vertical greens add up
• The result is a thick vertical green vector shown in fig.4.75(a) below:
 |
| Fig.4.75 |
Step 2:
(i) In this step, we add the two vectors shown in fig.4.75(a)
• Obviously, the resultant will be a vertical vector pointing upwards
(ii) This resultant vector is shown in magenta color in fig.4.75(b) above
7. So the magenta vector in fig.4.75(b) indicates the resultant dipole moment in NH3
• Scientists have determined it's value as 4.90 × 10-30 C m
• This value can be converted into Debye units
♦ We have: 1 D = 3.33564 × 10-30 C m
♦ ⇒ 1 C m = [1 ÷ (3.33564 × 10-30)] D
♦ ⇒ 4.90 × 10-30 C m = (4.90 × 10-30 ) × [1 ÷ (3.33564 × 10-30)] = 1.47 D
Dipole moment in NF3
• First we have to learn about the structure of a NF3 molecule. It is similar to that of NH3
• The only difference is that:
♦ In NH3, there is a H atom at each of the three corners of the base
♦ In NF3, there is a F atom at each of the three corners of the base
• So the final structure will be as shown in the fig.4.76(a) below. The wedge-dash-line model is shown in fig.b
 |
| Fig.4.76 |
So now we have an idea about the structure of the NF3 molecule
1. In this case, there are three bonds
♦ All of them are N-F bonds
♦ Those bonds do not lie on the same line
2. The orientations of the bonds can be described in two steps:
(i) The central atom N is situated at the apex of the triangular pyramid
(ii) The 3 bonds start from the N atom
♦ They are directed towards the corners of the 'base of the pyramid'
3. From the 3 bonds, we will have 3 dipole moments
• They are shown in yellow color in fig.4.77(a) below:
 |
| Fig.4.77 |
• Recall that, in the NH3 molecule, the dipole moments are due to the 'pulling of electrons' by the N atom
• So the 3 dipole moments in NF3 are opposite in directions when compared to NH3
4. In addition to the above 3 dipole moments, there is one more
• It is the same 'dipole moment due to lone pairs' that we saw in the case of NH3
• It is shown in orange color in the fig.4.77(a) above
5. So there are a total of 4 dipole moments
• But we want the dipole moment of the ‘molecule as a whole’
■ For that, we calculate the vector sum
• We have seen that, dipole moments are vector quantities
♦ So they can be added using 'principles of vector addition'
✰ Details can be seen here
6. We do the addition in two steps
• In step 1, we add the yellow vectors and find the resultant
• In step 2, we add the 'resultant obtained in step 1' with the orange vector
Step 1:
(i) To add the yellow vectors, we resolve them into rectangular components
• This is shown in fig.4.77(b) above
♦ The horizontal components are shown in red color
♦ The vertical components are shown in green color
(ii) The base triangle of the pyramid is an equilateral triangle
• So the horizontal reds pull symmetrically away from the center of the triangle
• This is shown in fig.4.77(c) above
• They cancel out giving a null vector
(iii) The vertical greens add up
• The result is a thick vertical green vector shown in fig.4.78(a) below:
 |
| Fig.4.78 |
(i) In this step, we add the two vectors shown in fig.4.78(a)
• Obviously, the resultant will be a vertical vector
(ii) But it's direction should be carefully determined
• The direction can be 'vertically upwards' or 'vertically downwards'
(iii) In fig.4.78(a), we have two vectors:
• The green and the orange
♦ They act in opposite directions
♦ The green is larger in magnitude
• So the resultant of green and orange will be acting downwards
(ii) This resultant vector is shown in magenta color in fig.4.78(b) above
7. So the magenta vector in fig.4.78(b) indicates the resultant dipole moment in NF3
• Scientists have determined it's value as 0.80 × 10-30 C m
• This value can be converted into Debye units
♦ We have: 1 D = 3.33564 × 10-30 C m
♦ ⇒ 1 C m = [1 ÷ (3.33564 × 10-30)] D
♦ ⇒ 0.80 × 10-30 C m = (0.80 × 10-30 ) × [1 ÷ (3.33564 × 10-30)] = 0.23 D
We can write a comparison. It can be written in 4 steps:
1. Direction of the dipole moment due to lone pair
• In NH3, it is vertically upwards
• In NF3 also, it is vertically upwards
2. Direction of the dipole moment in the bonds
• In NH3, the resultant of these dipole moments is vertically upwards
• In NF3, the resultant of these dipole moments is vertically downwards
3. Magnitude of the Final resultant
• In NH3, the final resultant is obtained by addition
♦ We can say:
✰ The dipole moment due to lone pair
✰ enhances the effect of
✰ The resultant dipole moment of the three N-H bonds
• In NF3, the final resultant is obtained by subtraction
♦ We can say:
✰ The dipole moment due to lone pair
✰ decreases the effect of
✰ The resultant dipole moment of the three N-F bonds
• So the magnitude of the final resultant will be higher in NH3
4. Direction of the Final resultant
• In NH3, the final resultant is vertically upwards
• In NF3, the final resultant is vertically downwards
1. Direction of the dipole moment due to lone pair
• In NH3, it is vertically upwards
• In NF3 also, it is vertically upwards
2. Direction of the dipole moment in the bonds
• In NH3, the resultant of these dipole moments is vertically upwards
• In NF3, the resultant of these dipole moments is vertically downwards
3. Magnitude of the Final resultant
• In NH3, the final resultant is obtained by addition
♦ We can say:
✰ The dipole moment due to lone pair
✰ enhances the effect of
✰ The resultant dipole moment of the three N-H bonds
• In NF3, the final resultant is obtained by subtraction
♦ We can say:
✰ The dipole moment due to lone pair
✰ decreases the effect of
✰ The resultant dipole moment of the three N-F bonds
• So the magnitude of the final resultant will be higher in NH3
4. Direction of the Final resultant
• In NH3, the final resultant is vertically upwards
• In NF3, the final resultant is vertically downwards
In the next section, we will see partial covalent character in ionic bonds
No comments:
Post a Comment