In the previous section, we saw the steps to find the formal charge. In this section, we will see Limitations of the octet rule
■ Recall that, the octet rule states:
Atoms can combine either by transfer of electrons from one atom to another or by sharing of valence electrons in order to have an octet in their valence shells
• We have learnt the details of the rule. But there are three points to note:
(i) Those details that we learnt can be used effectively for understanding the structures of organic compounds
(ii) Unfortunately, the successful application of octet rule is possible only in the case of the ‘elements of the 2nd period’
• That is., whenever two elements of the 2nd period combine with each other, we can draw the ‘Lewis dot structure’ based on the octet rule
(iii) From the 3rd period onwards, the ‘electrons in the d orbitals’ also may take part in the chemical reactions. In such cases, we will not be able to apply the octet rule
There are exceptions to the octet rule
■ What are those exceptions?
Answer:
There are three types of exceptions to the octet rule. They are:
(i) The incomplete octet of the central atom
(ii) Odd-electron molecules
(iii) The expanded octet
We will now see each of them in detail:
Example 1:
1. Fig.4.23 below shows the formation of LiCl (Lithium chloride)
♦ On the left side of the arrow, we see the Lewis symbols of Li and Cl
✰ Li has only one valence electron
♦ On the right side of the arrow, we see the Lewis dot structure of LiCl
• We notice that:
♦ Cl has attained octet
♦ But Li has not attained octet
✰ It needs 6 more electrons for octet
• Even with the 'Li without octet', the LiCl is a stable molecule
• The octet rule says that, atoms 'enter into bonds' to attain stable configuration (octet)
♦ But here, the Li enters into a bonding with Cl, even when it does not attain octet
■ So the octet rule fails to explain the formation of this molecule
Example 2:
1. Fig.4.24 below shows the formation of BeH2 (Beryllium dihydride)
♦ On the left side of the arrow, we see the Lewis symbols of H and Be
✰ Be has only two valence electrons
♦ On the right side of the arrow, we see the Lewis dot structure of BeH2
• We notice that:
♦ Both H atoms have attained octet
♦ But Be has not attained octet
✰ It needs 4 more electrons for octet
• Even with the 'Be without octet', the BeH2 is a stable molecule
• The octet rule says that, atoms 'enter into bonds' to attain stable configuration (octet)
♦ But here, the Be enters into a bonding with H, even when it does not attain octet
■ So the octet rule fails to explain the formation of this molecule
Example 3:
1. Fig.4.25 below shows the formation of BCl3 (Boron trichloride)
♦ On the left side of the arrow, we see the Lewis symbols of Cl and B
✰ B has only three valence electrons
♦ On the right side of the arrow, we see the Lewis dot structure of BCl3
• We notice that:
♦ All Cl atoms have attained octet
♦ But B has not attained octet
✰ It needs 2 more electrons for octet
• Even with the 'B without octet', the BCl3 is a stable molecule
• The octet rule says that, atoms 'enter into bonds' to attain stable configuration (octet)
♦ But here, the B enters into a bonding with Cl, even when it does not attain octet
■ So the octet rule fails to explain the formation of this molecule
• NO (Nitric oxide) is an example. It is shown in fig.4.26 below:
♦ On the left side of the arrow, we see the Lewis symbols of N and O
♦ On the right side of the arrow, we see the Lewis dot structure of NO
• We notice that:
♦ The O atom has attained octet
♦ But N has not attained octet
✰ It needs 1 more electron for octet
• Even with the 'N without octet', the NO is a stable molecule
• The octet rule says that, atoms 'enter into bonds' to attain stable configuration (octet)
♦ But here, the B enters into a bonding with Cl, even when it does not attain octet
■ So the octet rule fails to explain the formation of this molecule
Note that, the sum of valence electrons, which is [(5+6) = 11], is an odd number
(i) We know that, from the 3rd period onwards, the elements can possess the 3d orbitals also
(ii) The general electronic configuration of elements are in the pattern: 1s22s22p63s23p63d104s . . .
(iii) Note the terms with coefficient '2'. They are: 2s2 and 2p6
♦ From those two sub-shells, we get 8 electrons
(iv) But from the 3rd period onwards, d orbitals can also be present
♦ Some of the electrons in those 3d orbitals may take part in chemical bondings
(v) In such cases, we will see more than eight electrons around the central atom
■ This is called the expanded octet
(vi) 'oct'et is related to eight
• If there are more than eight electrons, it is obvious that, octet rule is not applicable
Let us see some examples:
Example 1:
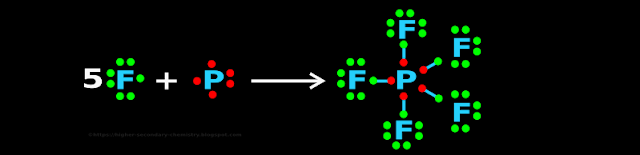
1. Fig.4.27 below shows the formation of PF5 (Phosphorus pentafluoride)
♦ On the left side of the arrow, we see the Lewis symbols of F and P
✰ F has seven valence electrons. It needs one more electron
✰ P has five valence electrons. It needs three more electrons
♦ On the right side of the arrow, we see the Lewis dot structure of PF5
• We notice that:
♦ All F atoms have attained octet
♦ The P atom needed only 3 more electrons. But it has acquired 5 more
✰ If it had attained 3 more, the total would have been (5+3) = 8
✰ But since it had attained 5 more, the total now is (5+5) = 10
• Compare this situation with N atom (belonging to the same group as P)
♦ N atom also needs 3 more electrons
♦ It can never acquire '5 more' because, there are no d orbitals available
■ So P atom has an expanded octet
Example 2:
1. Fig.4.28 below shows the formation of SF6 (Sulfur hexafluoride)
♦ On the left side of the arrow, we see the Lewis symbols of F and S
✰ F has seven valence electrons. It needs one more electron
✰ S has six valence electrons. It needs three more electrons
♦ On the right side of the arrow, we see the Lewis dot structure of SF6
• We notice that:
♦ All F atoms have attained octet
♦ The S atom needed only 2 more electrons. But it acquired 6 more
✰ If it had attained 2 more, the total would have been (6+2) = 8
✰ But since it had attained 6 more, the total now is (6+6) = 12
• Compare this situation with O atom (belonging to the same group as S)
♦ O atom also needs 2 more electrons
♦ It can never acquire '6 more' because, there are no d orbitals available
■ So S atom has an expanded octet
Example 3:
1. Fig.4.29 below shows the formation of H2SO4 (Sulfuric acid)
♦ On the left side of the arrow, we see the Lewis symbols of H, O and S
✰ H has one valence electron. It needs one more electron
✰ O has six valence electrons. It needs two more electrons
✰ S has six valence electrons. It needs two more electrons
♦ On the right side of the arrow, we see the Lewis dot structure of H2SO4
• We notice that:
♦ All H atoms have attained duplet
♦ All O atoms have attained octet
♦ The S atom needed only 2 more electrons. But it acquired 6 more
✰ If it had attained 2 more, the total would have been (6+2) = 8
✰ But since it had attained 6 more, the total now is (6+6) = 12
• Compare this situation with O atom (belonging to the same group as S)
♦ O atom also needs 2 more electrons
♦ It can never acquire '6 more' because, there are no d orbitals available
■ So S atom has an expanded octet
1. Fig.4.30 below shows the formation of SCl2 (Sulfur dichloride)
♦ On the left side of the arrow, we see the Lewis symbols of Cl and S
✰ Cl has seven valence electrons. It needs one more electron
✰ S has six valence electrons. It needs two more electrons
♦ On the right side of the arrow, we see the Lewis dot structure of SCl2
• We notice that:
♦ All Cl atoms have attained octet
♦ The S atom needed only 2 more electrons. Indeed, it has acquired only 2
■ So S atom obeys the octet rule in this case
■ Recall that, the octet rule states:
Atoms can combine either by transfer of electrons from one atom to another or by sharing of valence electrons in order to have an octet in their valence shells
• We have learnt the details of the rule. But there are three points to note:
(i) Those details that we learnt can be used effectively for understanding the structures of organic compounds
(ii) Unfortunately, the successful application of octet rule is possible only in the case of the ‘elements of the 2nd period’
• That is., whenever two elements of the 2nd period combine with each other, we can draw the ‘Lewis dot structure’ based on the octet rule
(iii) From the 3rd period onwards, the ‘electrons in the d orbitals’ also may take part in the chemical reactions. In such cases, we will not be able to apply the octet rule
■ So we can write:
■ What are those exceptions?
Answer:
There are three types of exceptions to the octet rule. They are:
(i) The incomplete octet of the central atom
(ii) Odd-electron molecules
(iii) The expanded octet
We will now see each of them in detail:
The incomplete octet of the central atom
This exception happens when the central atom has less than 4 valence electrons. It can be explained using some examples:Example 1:
1. Fig.4.23 below shows the formation of LiCl (Lithium chloride)
♦ On the left side of the arrow, we see the Lewis symbols of Li and Cl
✰ Li has only one valence electron
♦ On the right side of the arrow, we see the Lewis dot structure of LiCl
 |
| Fig.4.23 |
♦ Cl has attained octet
♦ But Li has not attained octet
✰ It needs 6 more electrons for octet
• Even with the 'Li without octet', the LiCl is a stable molecule
• The octet rule says that, atoms 'enter into bonds' to attain stable configuration (octet)
♦ But here, the Li enters into a bonding with Cl, even when it does not attain octet
■ So the octet rule fails to explain the formation of this molecule
Example 2:
1. Fig.4.24 below shows the formation of BeH2 (Beryllium dihydride)
♦ On the left side of the arrow, we see the Lewis symbols of H and Be
✰ Be has only two valence electrons
♦ On the right side of the arrow, we see the Lewis dot structure of BeH2
 |
| Fig.2.24 |
♦ Both H atoms have attained octet
♦ But Be has not attained octet
✰ It needs 4 more electrons for octet
• Even with the 'Be without octet', the BeH2 is a stable molecule
• The octet rule says that, atoms 'enter into bonds' to attain stable configuration (octet)
♦ But here, the Be enters into a bonding with H, even when it does not attain octet
■ So the octet rule fails to explain the formation of this molecule
Example 3:
1. Fig.4.25 below shows the formation of BCl3 (Boron trichloride)
♦ On the left side of the arrow, we see the Lewis symbols of Cl and B
✰ B has only three valence electrons
♦ On the right side of the arrow, we see the Lewis dot structure of BCl3
 |
| Fig.4.25 |
♦ All Cl atoms have attained octet
♦ But B has not attained octet
✰ It needs 2 more electrons for octet
• Even with the 'B without octet', the BCl3 is a stable molecule
• The octet rule says that, atoms 'enter into bonds' to attain stable configuration (octet)
♦ But here, the B enters into a bonding with Cl, even when it does not attain octet
■ So the octet rule fails to explain the formation of this molecule
Odd-electron molecules
• In some molecules, the 'sum of valence electrons of the atoms' will be an odd number. In such molecules, the octet rule will not be satisfied• NO (Nitric oxide) is an example. It is shown in fig.4.26 below:
 |
| Fig.4.26 |
♦ On the right side of the arrow, we see the Lewis dot structure of NO
♦ The O atom has attained octet
♦ But N has not attained octet
✰ It needs 1 more electron for octet
• Even with the 'N without octet', the NO is a stable molecule
• The octet rule says that, atoms 'enter into bonds' to attain stable configuration (octet)
♦ But here, the B enters into a bonding with Cl, even when it does not attain octet
■ So the octet rule fails to explain the formation of this molecule
Note that, the sum of valence electrons, which is [(5+6) = 11], is an odd number
The expanded octet
This can be explained in 6 steps:(i) We know that, from the 3rd period onwards, the elements can possess the 3d orbitals also
(ii) The general electronic configuration of elements are in the pattern: 1s22s22p63s23p63d104s . . .
(iii) Note the terms with coefficient '2'. They are: 2s2 and 2p6
♦ From those two sub-shells, we get 8 electrons
(iv) But from the 3rd period onwards, d orbitals can also be present
♦ Some of the electrons in those 3d orbitals may take part in chemical bondings
(v) In such cases, we will see more than eight electrons around the central atom
■ This is called the expanded octet
(vi) 'oct'et is related to eight
• If there are more than eight electrons, it is obvious that, octet rule is not applicable
Let us see some examples:
Example 1:
1. Fig.4.27 below shows the formation of PF5 (Phosphorus pentafluoride)
♦ On the left side of the arrow, we see the Lewis symbols of F and P
✰ F has seven valence electrons. It needs one more electron
✰ P has five valence electrons. It needs three more electrons
♦ On the right side of the arrow, we see the Lewis dot structure of PF5
 |
| Fig.4.27 |
♦ All F atoms have attained octet
♦ The P atom needed only 3 more electrons. But it has acquired 5 more
✰ If it had attained 3 more, the total would have been (5+3) = 8
✰ But since it had attained 5 more, the total now is (5+5) = 10
• Compare this situation with N atom (belonging to the same group as P)
♦ N atom also needs 3 more electrons
♦ It can never acquire '5 more' because, there are no d orbitals available
■ So P atom has an expanded octet
Example 2:
1. Fig.4.28 below shows the formation of SF6 (Sulfur hexafluoride)
♦ On the left side of the arrow, we see the Lewis symbols of F and S
✰ F has seven valence electrons. It needs one more electron
✰ S has six valence electrons. It needs three more electrons
♦ On the right side of the arrow, we see the Lewis dot structure of SF6
 |
| Fig.4.28 |
♦ All F atoms have attained octet
♦ The S atom needed only 2 more electrons. But it acquired 6 more
✰ If it had attained 2 more, the total would have been (6+2) = 8
✰ But since it had attained 6 more, the total now is (6+6) = 12
• Compare this situation with O atom (belonging to the same group as S)
♦ O atom also needs 2 more electrons
♦ It can never acquire '6 more' because, there are no d orbitals available
■ So S atom has an expanded octet
Example 3:
1. Fig.4.29 below shows the formation of H2SO4 (Sulfuric acid)
♦ On the left side of the arrow, we see the Lewis symbols of H, O and S
✰ H has one valence electron. It needs one more electron
✰ O has six valence electrons. It needs two more electrons
✰ S has six valence electrons. It needs two more electrons
♦ On the right side of the arrow, we see the Lewis dot structure of H2SO4
 |
| Fig.4.29 |
♦ All H atoms have attained duplet
♦ All O atoms have attained octet
♦ The S atom needed only 2 more electrons. But it acquired 6 more
✰ If it had attained 2 more, the total would have been (6+2) = 8
✰ But since it had attained 6 more, the total now is (6+6) = 12
• Compare this situation with O atom (belonging to the same group as S)
♦ O atom also needs 2 more electrons
♦ It can never acquire '6 more' because, there are no d orbitals available
■ So S atom has an expanded octet
However, S is a special case. It does obey octet rule in many compounds. An example is given below:
♦ On the left side of the arrow, we see the Lewis symbols of Cl and S
✰ Cl has seven valence electrons. It needs one more electron
✰ S has six valence electrons. It needs two more electrons
♦ On the right side of the arrow, we see the Lewis dot structure of SCl2
 |
| Fig.2.29 |
♦ All Cl atoms have attained octet
♦ The S atom needed only 2 more electrons. Indeed, it has acquired only 2
■ So S atom obeys the octet rule in this case
■ Note: The 'expanded octet' needs more detailed study. We want to know 'how the d orbitals get into bonds' with other atoms. We will see those details in later sections
Other drawbacks of the octet rule
1. The octet rule is based on the chemical inertness of noble gases
• That is., octet rule says that:
Every element tries to attain the stable electronic configuration of the nearest noble gas
• So the octet rule assumes that, the noble gases does not take part in chemical reactions because, they already have octet
• But in reality, some noble gases do react with elements like oxygen and fluorine
♦ Examples are: XeF2, KrF2, XeOF2 etc.,
• The octet rule is unable to give an explanation for such reactions
2. The molecules that we see around us have different shapes
♦ Some have planar shapes
♦ Some have pyramidal shapes
♦ So on . . .
• The octet rule is unable to give reasons for such different shapes
3. We know that elements react with each other to attain stability
• That means, molecules are more stable than atoms
• The octet rule is not able to give reasons for that ‘greater stability’
• This is mainly because, the octet rule is silent about ‘energy changes’ involved in reactions
1. The octet rule is based on the chemical inertness of noble gases
• That is., octet rule says that:
Every element tries to attain the stable electronic configuration of the nearest noble gas
• So the octet rule assumes that, the noble gases does not take part in chemical reactions because, they already have octet
• But in reality, some noble gases do react with elements like oxygen and fluorine
♦ Examples are: XeF2, KrF2, XeOF2 etc.,
• The octet rule is unable to give an explanation for such reactions
2. The molecules that we see around us have different shapes
♦ Some have planar shapes
♦ Some have pyramidal shapes
♦ So on . . .
• The octet rule is unable to give reasons for such different shapes
3. We know that elements react with each other to attain stability
• That means, molecules are more stable than atoms
• The octet rule is not able to give reasons for that ‘greater stability’
• This is mainly because, the octet rule is silent about ‘energy changes’ involved in reactions
In the next section, we will see ionic bonds
No comments:
Post a Comment