In the previous section 2.18, we saw the electronic configuration of atoms up to no.12 magnesium (Mg).In this section, we will see from no.13 on wards. We will use the shortened method using [Ne]. This is because, for every element which comes after neon, the configuration of neon will be readily present on the left end. There is no need to repeat that configuration every time.
■ We know that, the configuration of Ne is: 1s22s22p6
• If we see a configuration with [Ne], all we need to do is to put '1s22s22p6' in place of that [Ne]
• Similarly, if we see [Ne] in an orbital diagram, all we need to do, is to put the 'boxes of neon' in it's place
So we have completed the electronic configuration of (Mg). Next is aluminium (Al)
■ We know that, the configuration of Ne is: 1s22s22p6
• If we see a configuration with [Ne], all we need to do is to put '1s22s22p6' in place of that [Ne]
• Similarly, if we see [Ne] in an orbital diagram, all we need to do, is to put the 'boxes of neon' in it's place
So we have completed the electronic configuration of (Mg). Next is aluminium (Al)
1. Al has 13 electrons
• It’s first 12 electrons can go into the 1s, 2s, 2p and 3s orbitals just like in the previous Mg
• We have to find a place for the 13th electron
2. The 3s orbital is already filled up
• From the chart, we see that, the next higher energy level is 3p
• So the 13th electron will go into the 3px orbital
3. The electronic configuration of Al using the orbital diagram method is shown in fig.2.58 below
• The electronic configuration using s, p, d, f method is also shown. It is shown in green color
4. Starting with Al, six elements can be accommodated in the 3p sub-shell
• They are: Aluminium (Al), silicon (S), Phosphorus (P), Sulfur (S), Chlorine (Cl) and Argon (Ar)
(i) The 3p sub-shell has 3 degenerate orbitals: 3px, 3py and 3pz
(ii) We have to follow the Hund's rule
• This is similar to a situation that we saw in the previous section:
• The six elements from boron (B) to neon (Ne) were accommodated in the 2p sub-shell according to the Hund's rule
(iii) We can follow the same procedure here:
• First we obtain maximum number of half filled orbitals. Then we start pairing
• The six elements from Al (no.13) to Ar (no.18) are shown in the fig.2.58 above. We see that, the pairing starts from S (no.16)
• It’s first 12 electrons can go into the 1s, 2s, 2p and 3s orbitals just like in the previous Mg
• We have to find a place for the 13th electron
2. The 3s orbital is already filled up
• From the chart, we see that, the next higher energy level is 3p
• So the 13th electron will go into the 3px orbital
3. The electronic configuration of Al using the orbital diagram method is shown in fig.2.58 below
 |
| Fig.2.58 |
4. Starting with Al, six elements can be accommodated in the 3p sub-shell
• They are: Aluminium (Al), silicon (S), Phosphorus (P), Sulfur (S), Chlorine (Cl) and Argon (Ar)
(i) The 3p sub-shell has 3 degenerate orbitals: 3px, 3py and 3pz
(ii) We have to follow the Hund's rule
• This is similar to a situation that we saw in the previous section:
• The six elements from boron (B) to neon (Ne) were accommodated in the 2p sub-shell according to the Hund's rule
(iii) We can follow the same procedure here:
• First we obtain maximum number of half filled orbitals. Then we start pairing
• The six elements from Al (no.13) to Ar (no.18) are shown in the fig.2.58 above. We see that, the pairing starts from S (no.16)
So we have completed the electronic configuration up to Argon. Next is the element with atomic number 19, which is: potassium (K)
1. K has 19 electrons
• It’s first 18 electrons can go into the 1s, 2s, 2p, 3s and 3p orbitals just like in the previous Ar
• We have to find a place for the 19th electron
2. All the orbitals of 3p sub-shell are filled up with argon
• From the chart, we see that, the next higher energy level is 4s
(Note that, after 3p, the next higher is 4s. It is not 3d)
3. In the 4s, there is only one orbital
• That orbital can hold two electrons
• So the 19th electron of K will go into 4s
4. So the electronic configuration of K will be: [Ne]3s23p64s1
• Consider the portion to the left of 4s1.
♦ It is [Ne]3s23p6
• But [Ne]3s23p6 is [Ar]
• So the electronic configuration of K will be [Ar]4s1
5. The orbital diagram and the electronic configuration are shown in fig.2.58 above
1. K has 19 electrons
• It’s first 18 electrons can go into the 1s, 2s, 2p, 3s and 3p orbitals just like in the previous Ar
• We have to find a place for the 19th electron
2. All the orbitals of 3p sub-shell are filled up with argon
• From the chart, we see that, the next higher energy level is 4s
(Note that, after 3p, the next higher is 4s. It is not 3d)
3. In the 4s, there is only one orbital
• That orbital can hold two electrons
• So the 19th electron of K will go into 4s
4. So the electronic configuration of K will be: [Ne]3s23p64s1
• Consider the portion to the left of 4s1.
♦ It is [Ne]3s23p6
• But [Ne]3s23p6 is [Ar]
• So the electronic configuration of K will be [Ar]4s1
5. The orbital diagram and the electronic configuration are shown in fig.2.58 above
So we have completed the electronic configuration up to K. Next is the element with atomic number 20, which is: calcium (Ca)
1. Ca has 20 electrons
• It’s first 19 electrons can go into the 1s, 2s, 2p, 3s, 3p and 4s orbitals just like in the previous K
• We have to find a place for the 20th electron
2. The 4s orbital can hold 2 electrons
• So the 20th electron will go into the 4s orbital
3. The orbital diagram and the electronic configuration are shown in fig.2.58 above
So we have completed the electronic configuration of Ca (no.20). Next is the element with atomic number 21, which is: scandium (Sc)
1. Sc has 21 electrons
• So the 21st electron will go into the $\mathbf\small{3d_{xy}}$ orbital
3. The electronic configuration of Sc using the orbital diagram method is shown in fig.2.59 below:
• The electronic configuration using s, p, d, f method is also shown. It is shown in green color
4. Starting with Sc, ten elements can be accommodated in the 3d sub-shell
• They are:
21 (Sc) Scandium, 22 (Ti) Titanium, 23 (V) Vanadium, 24 (Cr) Chromium 25 (Mn) Manganese
26 (Fe) Iron, 27 (Co) Cobalt, 28 (Ni) Nickel, 29 (Cu) Copper, 30 (Zn) Zinc, 31 (Ga) Gallium
(i) The 3d sub-shell has 5 degenerate orbitals: $\mathbf\small{d_{xy},\,d_{xz},\,d_{yz},\,d_{x^2-y^2},\,d_{z^2}}$
(ii) We have to follow the Hund's rule
• This is similar to a situation that we saw in the previous section:
• The six elements from boron (B) to neon (Ne) were accommodated in the 2p sub-shell according to the Hund's rule
• We saw it again in the case of elements from Al (no.13) to Ar (no.18)
(iii) We can follow the same procedure here:
• First we obtain maximum number of half filled orbitals. Then we start pairing
• The ten elements from Sc (no.21) to Ga (no.31) are shown in the fig.2.58 above. We see that, the pairing starts from Fe (no.26)
5. We see an unusual situation in Cr (no.24)
Let us see what exactly it is:
(i) From Sc (no.21) onwards, the electrons begin to enter the 3d orbitals
(ii) In Cr (no.24), the 24th electron enters the 4th 3d orbital
• When this 24th electron has settled inside that 4th 3d orbital, the Sc atom sees an opportunity
• An opportunity to attain more stability
(iii) The Sc atom realizes that, if one of the electrons in the 4s can be transferred to the 3d orbital, all the 3d orbitals will become half filled
■ Half filled and fully filled orbitals have more stability
(iv) So one of the electrons in the 4s orbital jumps to the 3d orbital
• This is indicated by the white curved arrow
(v) The expected configuration is shown inside a magenta box. It is a wrong configuration
• The actual configuration is written below the magenta box
(vi) Note that, the 'opportunity' to attain half filled orbitals becomes available only when the 24th electron enters the atom. No such opportunity is available before that
• That means, no such opportunity is available before chromium
(vii) Note that, after Cr, the 25th electron in Mn (no.25) goes to the 4s orbital because, that 4s is the lowest energy orbital available to Mn
6. We see a similar unusual situation in Cu (no.29)
Let us see what exactly it is:
(i) From Fe (no.26) onwards, the pairing of electrons begins in the 3d orbitals
(ii) In Cu (no.29), the 29th electron enters the 4th 3d orbital
• When this 29th electron has settled inside that 4th 3d orbital, the Cu atom sees an opportunity
• An opportunity to attain more stability
(iii) The Cu atom realizes that, if one of the electrons in the 4s can be transferred to the 3d orbital, all the 3d orbitals will become fully filled
■ As we have seen above, half filled and fully filled orbitals have more stability
(iv) So one of the electrons in the 4s orbital jumps to the 3d orbital
• This is indicated by the white curved arrow
(v) The expected configuration is shown inside a magenta box. It is a wrong configuration
• The actual configuration is written below the magenta box
(vi) Note that, the 'opportunity' to attain fully filed orbitals becomes available only when the 29th electron enters the atom. No such opportunity is available before that
• That means, no such opportunity is available before copper
(vii) Note that, after Cu, the 30th electron in Zn (no.30) goes to the 4s orbital because, that 4s is the lowest energy orbital available to Zn
• The only two differences are at Cr (no.24) and Cu (no.29)
1. Ca has 20 electrons
• It’s first 19 electrons can go into the 1s, 2s, 2p, 3s, 3p and 4s orbitals just like in the previous K
• We have to find a place for the 20th electron
2. The 4s orbital can hold 2 electrons
• So the 20th electron will go into the 4s orbital
3. The orbital diagram and the electronic configuration are shown in fig.2.58 above
So we have completed the electronic configuration of Ca (no.20). Next is the element with atomic number 21, which is: scandium (Sc)
1. Sc has 21 electrons
• It’s first 20 electrons can go into the 1s, 2s, 2p, 3s, 3p and 4s orbitals just like in the previous Ca
• We have to find a place for the 21th electron
• From the chart, we see that, the next higher energy level is 3d • We have to find a place for the 21th electron
2. The 4s orbital is already filled up
• So the 21st electron will go into the $\mathbf\small{3d_{xy}}$ orbital
3. The electronic configuration of Sc using the orbital diagram method is shown in fig.2.59 below:
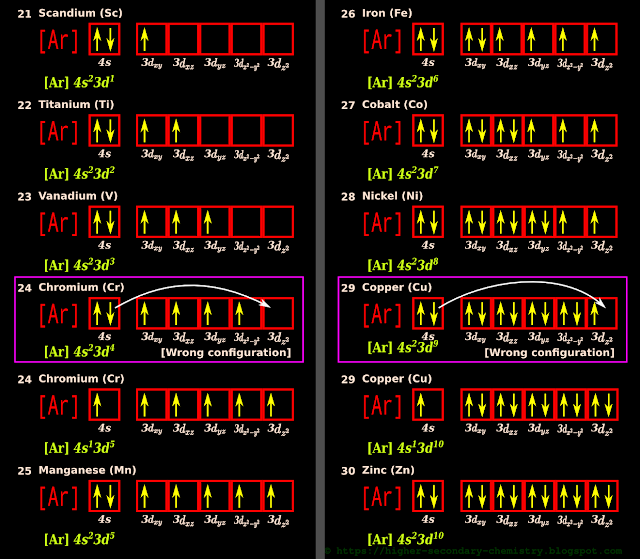 |
| Fig.2.59 |
4. Starting with Sc, ten elements can be accommodated in the 3d sub-shell
• They are:
21 (Sc) Scandium, 22 (Ti) Titanium, 23 (V) Vanadium, 24 (Cr) Chromium 25 (Mn) Manganese
26 (Fe) Iron, 27 (Co) Cobalt, 28 (Ni) Nickel, 29 (Cu) Copper, 30 (Zn) Zinc, 31 (Ga) Gallium
(i) The 3d sub-shell has 5 degenerate orbitals: $\mathbf\small{d_{xy},\,d_{xz},\,d_{yz},\,d_{x^2-y^2},\,d_{z^2}}$
(ii) We have to follow the Hund's rule
• This is similar to a situation that we saw in the previous section:
• The six elements from boron (B) to neon (Ne) were accommodated in the 2p sub-shell according to the Hund's rule
• We saw it again in the case of elements from Al (no.13) to Ar (no.18)
(iii) We can follow the same procedure here:
• First we obtain maximum number of half filled orbitals. Then we start pairing
• The ten elements from Sc (no.21) to Ga (no.31) are shown in the fig.2.58 above. We see that, the pairing starts from Fe (no.26)
5. We see an unusual situation in Cr (no.24)
Let us see what exactly it is:
(i) From Sc (no.21) onwards, the electrons begin to enter the 3d orbitals
(ii) In Cr (no.24), the 24th electron enters the 4th 3d orbital
• When this 24th electron has settled inside that 4th 3d orbital, the Sc atom sees an opportunity
• An opportunity to attain more stability
(iii) The Sc atom realizes that, if one of the electrons in the 4s can be transferred to the 3d orbital, all the 3d orbitals will become half filled
■ Half filled and fully filled orbitals have more stability
(iv) So one of the electrons in the 4s orbital jumps to the 3d orbital
• This is indicated by the white curved arrow
(v) The expected configuration is shown inside a magenta box. It is a wrong configuration
• The actual configuration is written below the magenta box
(vi) Note that, the 'opportunity' to attain half filled orbitals becomes available only when the 24th electron enters the atom. No such opportunity is available before that
• That means, no such opportunity is available before chromium
(vii) Note that, after Cr, the 25th electron in Mn (no.25) goes to the 4s orbital because, that 4s is the lowest energy orbital available to Mn
6. We see a similar unusual situation in Cu (no.29)
Let us see what exactly it is:
(i) From Fe (no.26) onwards, the pairing of electrons begins in the 3d orbitals
(ii) In Cu (no.29), the 29th electron enters the 4th 3d orbital
• When this 29th electron has settled inside that 4th 3d orbital, the Cu atom sees an opportunity
• An opportunity to attain more stability
(iii) The Cu atom realizes that, if one of the electrons in the 4s can be transferred to the 3d orbital, all the 3d orbitals will become fully filled
■ As we have seen above, half filled and fully filled orbitals have more stability
(iv) So one of the electrons in the 4s orbital jumps to the 3d orbital
• This is indicated by the white curved arrow
(v) The expected configuration is shown inside a magenta box. It is a wrong configuration
• The actual configuration is written below the magenta box
(vi) Note that, the 'opportunity' to attain fully filed orbitals becomes available only when the 29th electron enters the atom. No such opportunity is available before that
• That means, no such opportunity is available before copper
(vii) Note that, after Cu, the 30th electron in Zn (no.30) goes to the 4s orbital because, that 4s is the lowest energy orbital available to Zn
7. So it is smooth sailing for the ten elements from Sc (no.21) to Zn (no.30)
• They strictly follow the 'Hund's rule' and 'relative orbital energy rule'• The only two differences are at Cr (no.24) and Cu (no.29)
So we have completed the electronic configuration up to Zinc (no.30). Next is the element with atomic number 31, which is: gallium (Ga)
1. Ga has 31 electrons
• It’s first 30 electrons can go into the 1s, 2s, 2p, 3s, 3d and 4s orbitals just like in the previous Zn
• We have to find a place for the 31st electron
2. The 4s and 3d orbitals are already filled up
• From the chart, we see that, the next higher energy level is 4p
• So the 31st electron will go into the 4px orbital
3. The electronic configuration of Ga using the orbital diagram method is shown in fig.2.60 below:
• The electronic configuration using s, p, d, f method is also shown. It is shown in green color
4. Starting with Ga, six elements can be accommodated in the 4p sub-shell
• They are: 31 Gallium (Ga), 32 Germanium (Ge), 33 Arsenic (As), 34 Selenium (Se),
35 Bromine (Br), 36 Krypton (Kr)
(i) The 3p sub-shell has 3 degenerate orbitals: 3px, 3py and 3pz
(ii) We have to follow the Hund's rule
• This is similar to a situation that we saw in the previous section:
• The six elements from boron (B) to neon (Ne) were accommodated in the 2p sub-shell according to the Hund's rule
(iii) We can follow the same procedure here:
• First we obtain maximum number of half filled orbitals. Then we start pairing
• The six elements from Ga (no.31) to Kr (no.36) are shown in the fig.2.60 above. We see that, the pairing starts from Se (no.34)
• It’s first 30 electrons can go into the 1s, 2s, 2p, 3s, 3d and 4s orbitals just like in the previous Zn
• We have to find a place for the 31st electron
2. The 4s and 3d orbitals are already filled up
• From the chart, we see that, the next higher energy level is 4p
• So the 31st electron will go into the 4px orbital
3. The electronic configuration of Ga using the orbital diagram method is shown in fig.2.60 below:
 |
| Fig.2.60 |
4. Starting with Ga, six elements can be accommodated in the 4p sub-shell
• They are: 31 Gallium (Ga), 32 Germanium (Ge), 33 Arsenic (As), 34 Selenium (Se),
35 Bromine (Br), 36 Krypton (Kr)
(i) The 3p sub-shell has 3 degenerate orbitals: 3px, 3py and 3pz
(ii) We have to follow the Hund's rule
• This is similar to a situation that we saw in the previous section:
• The six elements from boron (B) to neon (Ne) were accommodated in the 2p sub-shell according to the Hund's rule
(iii) We can follow the same procedure here:
• First we obtain maximum number of half filled orbitals. Then we start pairing
• The six elements from Ga (no.31) to Kr (no.36) are shown in the fig.2.60 above. We see that, the pairing starts from Se (no.34)
So we have completed the electronic configuration up to krypton (no.36)
We will write a summary of the work we did until now
1. We start with 1H
• When 2He is reached, the 1s orbital is completely filled up
• This we saw in fig.2.53
■ 2He is a milestone because, all the orbitals in it are completely filled. And also the 1st main-shell is completely filled
2. Filling of 2s begins in 3Li
• When 4Be is reached, the 2s orbital is completely filled up
• This we saw in fig.2.55
3. Filling of 2p begins in 5B
• When 10Ne is reached, the 2p orbitals are completely filled up
• Note that, there are 6 elements from 5B to 10Ne
♦ Thus the 6 electrons in the 2p orbitals are filled up
• This we saw in fig.2.55
■ 10Ne is a milestone because, all the orbitals in it are completely filled. And also the 2nd main-shell is completely filled
4. Filling of 3s begins in 11Na
• When 12Mg is reached, the 3s orbital is completely filled up
• This we saw in fig.2.56
5. Filling of 3p begins in 13Al
• When 18Ar is reached, the 3p orbitals are completely filled up
• Note that, there are 6 elements from 13Al to 18Ar
♦ Thus the 6 electrons in the 3p orbitals are filled up
• This we saw in fig.2.58
■ 18Ar is a milestone because, all the orbitals in it are completely filled. And also the 3rd main-shell is completely filled
6. Filling of 4s begins in 19K
• When 20Ca is reached, the 4s orbital is completely filled up
• This we saw in fig.2.58
7. After 4s, the orbital with next higher energy is 3d
• Filling of 3d begins in 21Sc
• When 30Zn is reached, the 3d orbitals are completely filled up
• Note that, there are 10 elements from 21Sc to 30Zn
♦ Thus the 10 electrons in the 3d orbitals are filled up
• This we saw in fig.2.59
■ Also keep in mind the unusual situations in 24Cr and 29Cu
8. After 3d, the orbital with next higher energy is 4p
• Filling of 4p begins in 31Ga
• When 36Kr is reached, the 4p orbitals are completely filled up
• Note that, there are 6 elements from 31Ga to 36Kr
♦ Thus the 6 electrons in the 4p orbitals are filled up
• This we saw in fig.2.60
■ 36Kr is a milestone because, all the orbitals in it are completely filled
We will write a summary of the work we did until now
1. We start with 1H
• When 2He is reached, the 1s orbital is completely filled up
• This we saw in fig.2.53
■ 2He is a milestone because, all the orbitals in it are completely filled. And also the 1st main-shell is completely filled
2. Filling of 2s begins in 3Li
• When 4Be is reached, the 2s orbital is completely filled up
• This we saw in fig.2.55
3. Filling of 2p begins in 5B
• When 10Ne is reached, the 2p orbitals are completely filled up
• Note that, there are 6 elements from 5B to 10Ne
♦ Thus the 6 electrons in the 2p orbitals are filled up
• This we saw in fig.2.55
■ 10Ne is a milestone because, all the orbitals in it are completely filled. And also the 2nd main-shell is completely filled
4. Filling of 3s begins in 11Na
• When 12Mg is reached, the 3s orbital is completely filled up
• This we saw in fig.2.56
5. Filling of 3p begins in 13Al
• When 18Ar is reached, the 3p orbitals are completely filled up
• Note that, there are 6 elements from 13Al to 18Ar
♦ Thus the 6 electrons in the 3p orbitals are filled up
• This we saw in fig.2.58
■ 18Ar is a milestone because, all the orbitals in it are completely filled. And also the 3rd main-shell is completely filled
6. Filling of 4s begins in 19K
• When 20Ca is reached, the 4s orbital is completely filled up
• This we saw in fig.2.58
7. After 4s, the orbital with next higher energy is 3d
• Filling of 3d begins in 21Sc
• When 30Zn is reached, the 3d orbitals are completely filled up
• Note that, there are 10 elements from 21Sc to 30Zn
♦ Thus the 10 electrons in the 3d orbitals are filled up
• This we saw in fig.2.59
■ Also keep in mind the unusual situations in 24Cr and 29Cu
8. After 3d, the orbital with next higher energy is 4p
• Filling of 4p begins in 31Ga
• When 36Kr is reached, the 4p orbitals are completely filled up
• Note that, there are 6 elements from 31Ga to 36Kr
♦ Thus the 6 electrons in the 4p orbitals are filled up
• This we saw in fig.2.60
■ 36Kr is a milestone because, all the orbitals in it are completely filled
• So we have completed up to no.36 krypton. In the next section we will see the remaining elements
No comments:
Post a Comment